Boronic acid with high oxidative stability and utility in biological contexts.
Graham, B.J., Windsor, I.W., Gold, B., Raines, R.T.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33653951
- DOI: https://doi.org/10.1073/pnas.2013691118
- Primary Citation of Related Structures:
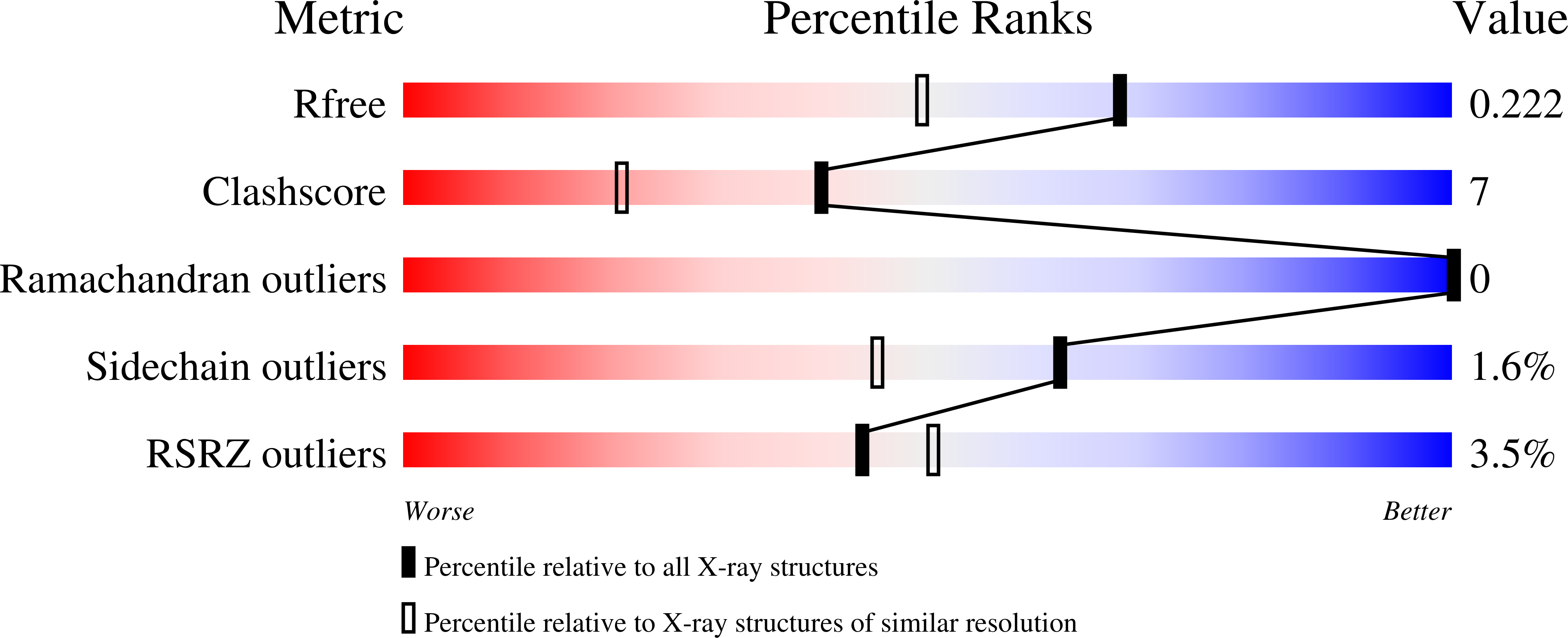
6U0Q - PubMed Abstract:
Despite their desirable attributes, boronic acids have had a minimal impact in biological contexts. A significant problem has been their oxidative instability. At physiological pH, phenylboronic acid and its boronate esters are oxidized by reactive oxygen species at rates comparable to those of thiols. After considering the mechanism and kinetics of the oxidation reaction, we reasoned that diminishing electron density on boron could enhance oxidative stability. We found that a boralactone, in which a carboxyl group serves as an intramolecular ligand for the boron, increases stability by 10 4 -fold. Computational analyses revealed that the resistance to oxidation arises from diminished stabilization of the p orbital of boron that develops in the rate-limiting transition state of the oxidation reaction. Like simple boronic acids and boronate esters, a boralactone binds covalently and reversibly to 1,2-diols such as those in saccharides. The kinetic stability of its complexes is, however, at least 20-fold greater. A boralactone also binds covalently to a serine side chain in a protein. These attributes confer unprecedented utility upon boralactones in the realms of chemical biology and medicinal chemistry.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139.