Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) with Specific Recognition for Cancer-Associated Glycans: Production, Structural Characterization, and Target Identification.
Martinez-Alarcon, D., Varrot, A., Fitches, E., Gatehouse, J.A., Cao, M., Pyati, P., Blanco-Labra, A., Garcia-Gasca, T.(2020) Biomolecules 10
- PubMed: 32340396
- DOI: https://doi.org/10.3390/biom10040654
- Primary Citation of Related Structures:
6TT9 - PubMed Abstract:
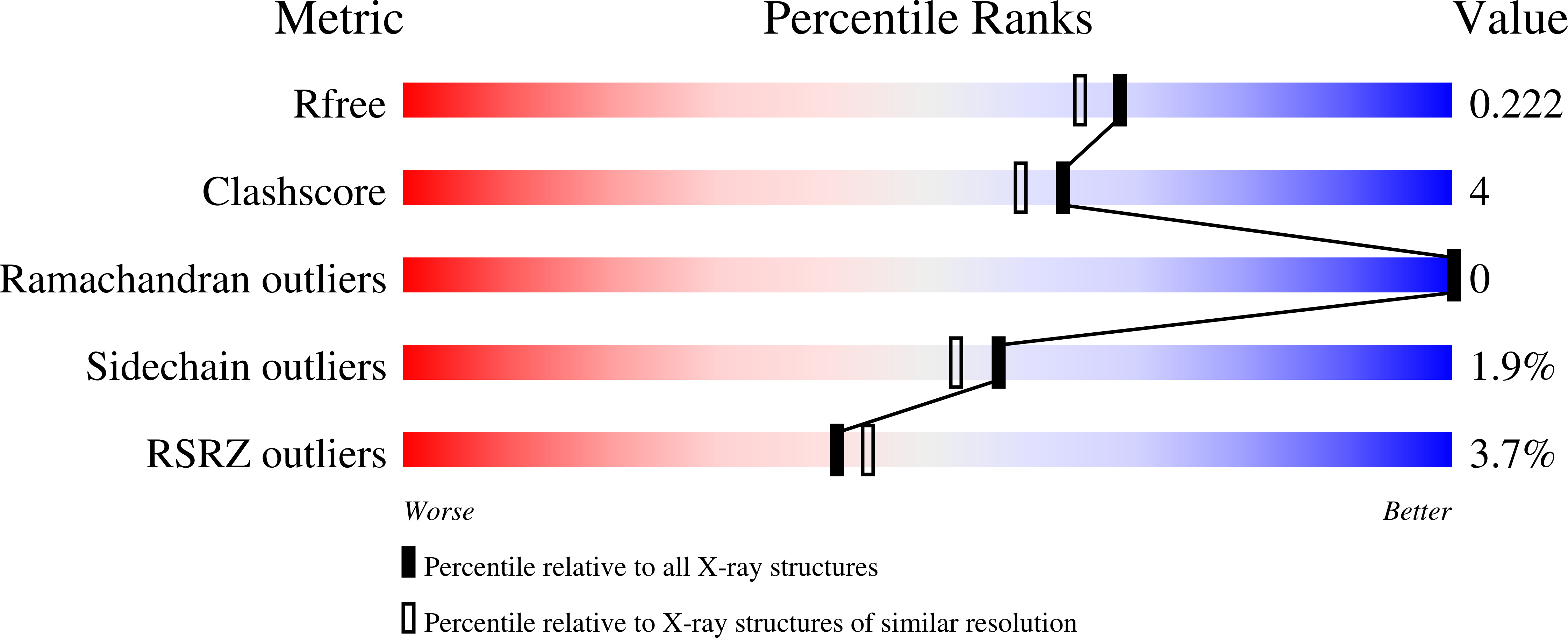
Herein, we report the production of a recombinant Tepary bean lectin ( r TBL-1), its three-dimensional (3D) structure, and its differential recognition for cancer-type glycoconjugates. r TBL-1 was expressed in Pichia pastoris, yielding 316 mg per liter of culture, and was purified by nickel affinity chromatography. Characterization of the protein showed that r TBL-1 is a stable 120 kDa homo-tetramer folded as a canonical leguminous lectin with two divalent cations (Ca 2+ and Mn 2+ ) attached to each subunit, confirmed in its 3D structure solved by X-ray diffraction at 1.9 Å resolution. Monomers also presented a ~2.5 kDa N -linked glycan located on the opposite face of the binding pocket. It does not participate in carbohydrate recognition but contributes to the stabilization of the interfaces between protomers. Screening for potential r TBL-1 targets by glycan array identified 14 positive binders, all of which correspond to β1-6 branched N -glycans' characteristics of cancer cells. The presence of α1-6 core fucose, also tumor-associated, improved carbohydrate recognition. r TBL-1 affinity for a broad spectrum of mono- and disaccharides was evaluated by isothermal titration calorimetry (ITC); however, no interaction was detected, corroborating that carbohydrate recognition is highly specific and requires larger ligands for binding. This would explain the differential recognition between healthy and cancer cells by Tepary bean lectins.
Organizational Affiliation:
Centro de Investigación y de Estudios Avanzados Unidad Irapuato, Departamento de Biotecnología y Bioquímica, Irapuato 36821, Guanaj uato, Mexico.