An assessment of the use of Hepatitis B Virus core protein virus-like particles to display heterologous antigens from Neisseria meningitidis.
Aston-Deaville, S., Carlsson, E., Saleem, M., Thistlethwaite, A., Chan, H., Maharjan, S., Facchetti, A., Feavers, I.M., Alistair Siebert, C., Collins, R.F., Roseman, A., Derrick, J.P.(2020) Vaccine 38: 3201-3209
- PubMed: 32178907
- DOI: https://doi.org/10.1016/j.vaccine.2020.03.001
- Primary Citation of Related Structures:
6TIK - PubMed Abstract:
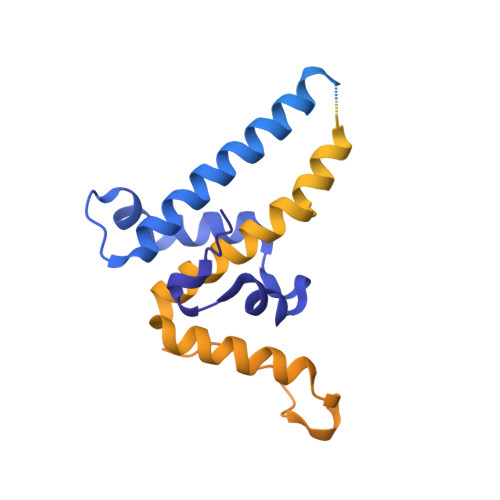
Neisseria meningitidis is the causative agent of meningococcal meningitis and sepsis and remains a significant public health problem in many countries. Efforts to develop a comprehensive vaccine against serogroup B meningococci have focused on the use of surface-exposed outer membrane proteins. Here we report the use of virus-like particles derived from the core protein of Hepatitis B Virus, HBc, to incorporate antigen domains derived from Factor H binding protein (FHbp) and the adhesin NadA. The extracellular domain of NadA was inserted into the major immunodominant region of HBc, and the C-terminal domain of FHbp at the C-terminus (CFHbp), creating a single polypeptide chain 3.7-fold larger than native HBc. Remarkably, cryoelectron microscopy revealed that the construct formed assemblies that were able to incorporate both antigens with minimal structural changes to native HBc. Electron density was weak for NadA and absent for CFHbp, partly attributable to domain flexibility. Following immunization of mice, three HBc fusions (CFHbp or NadA alone, NadA + CFHbp) were able to induce production of IgG1, IgG2a and IgG2b antibodies reactive against their respective antigens at dilutions in excess of 1:18,000. However, only HBc fusions containing NadA elicited the production of antibodies with serum bactericidal activity. It is hypothesized that this improved immune response is attributable to the adoption of a more native-like folding of crucial conformational epitopes of NadA within the chimeric VLP. This work demonstrates that HBc can incorporate insertions of large antigen domains but that maintenance of their three-dimensional structure is likely to be critical in obtaining a protective response.
Organizational Affiliation:
Lydia Becker Institute of Immunology and Inflammation, School of Biological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, University of Manchester, Manchester M13 9PL, UK.