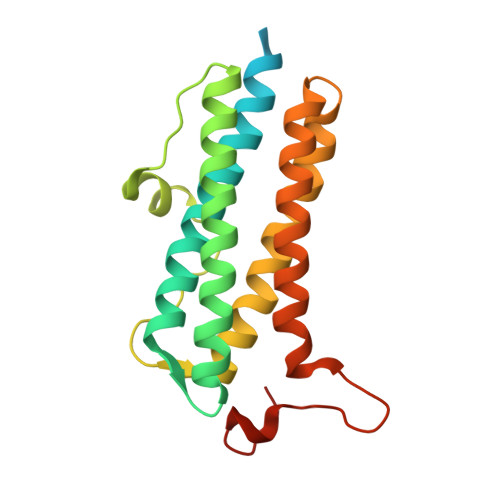
The crystal structure of the DPS2 from DEINOCOCCUS RADIODURANS to 1.83A resolution (sequentially soaked in CaCl2 [5mM] for 20 min, then in Ammonium iron(II) sulfate [10mM] for 2h).
Cuypers, M.G., McSweeney, S., Romao, C.V., Mitchell, E.P.To be published.