Sulfoxides of sulfur-containing amino acids are suicide substrates of Citrobacter freundii methionine gamma-lyase. Structural bases of the enzyme inactivation.
Revtovich, S., Morozova, E., Kulikova, V., Koval, V., Anufrieva, N., Nikulin, A., Demidkina, T.(2020) Biochimie 168: 190-197
- PubMed: 31711941
- DOI: https://doi.org/10.1016/j.biochi.2019.11.004
- Primary Citation of Related Structures:
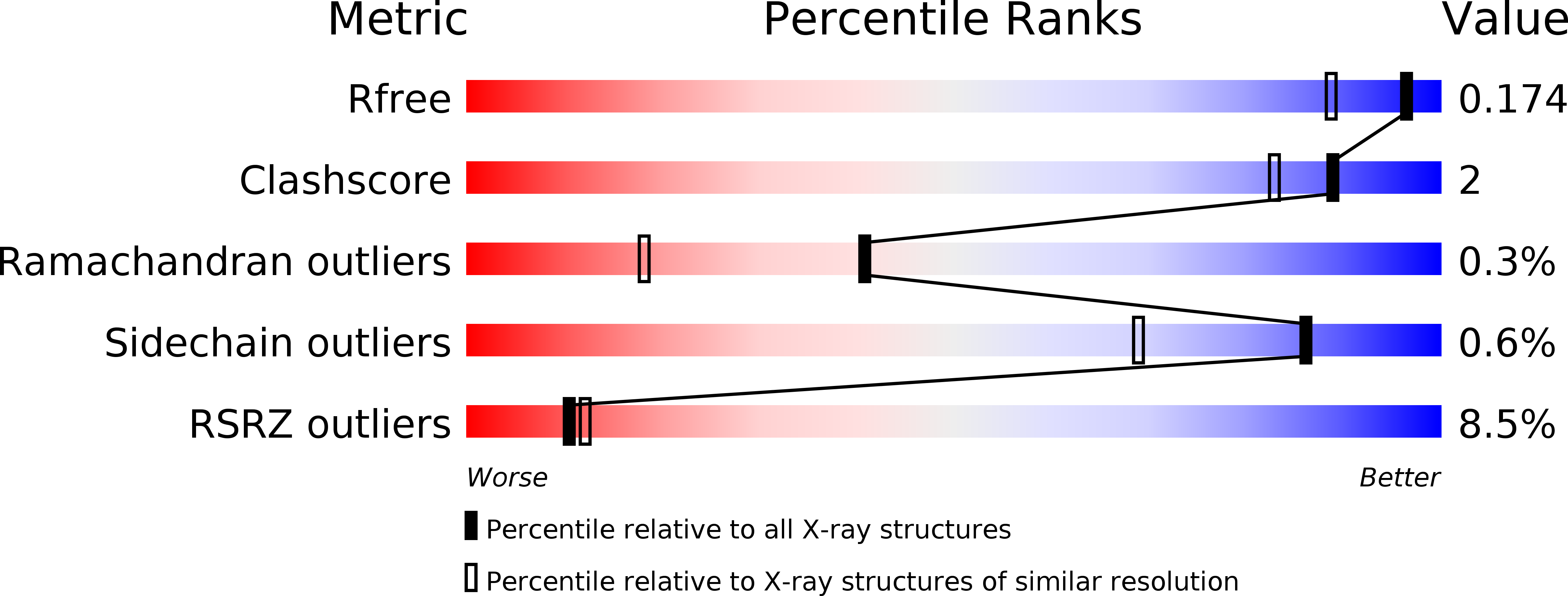
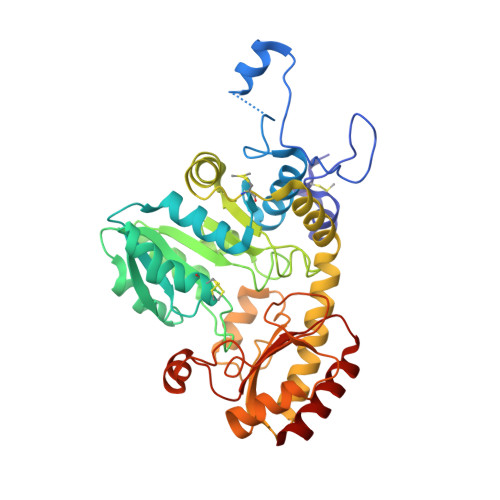
5K30, 6S0C - PubMed Abstract:
Interactions of Citrobacter freundii methionine γ-lyase (MGL) with sulfoxides of typical substrates were investigated. It was found that sulfoxides are suicide substrates of the enzyme. The products of the β- and γ-elimination reactions of sulfoxides, thiosulfinates, oxidize three cysteine residues of the enzyme. Three-dimensional structures of MGL inactivated by dimethyl thiosulfinate and diethyl thiosulfinate were determined at 1.46 Å and 1.59 Å resolution. Analysis of the structures identified SH groups oxidized by thiosulfinates and revealed the structural bases of MGL inactivation. The extent of inactivation of MGL in the catalysis of the β-elimination reaction depends on the length of the «tail» at oxidized Cys115. Oxidation of Cys115 results in MGL incapable to catalyze the stage of methyl mercaptan elimination of the physiological reaction.
Organizational Affiliation:
Engelhardt Institute of Molecular Biology of the Russian Academy of Sciences, Moscow, Russia. Electronic address: svetla21@mail.ru.