CRYSTAL STRUCTURE OF THE HUMAN PRMT5:MEP50 COMPLEX with JNJ45031882
Brown, D.G., Robinson, C.M., Pande, V.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein arginine N-methyltransferase 5 | 644 | Homo sapiens | Mutation(s): 0 Gene Names: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 EC: 2.1.1.320 | 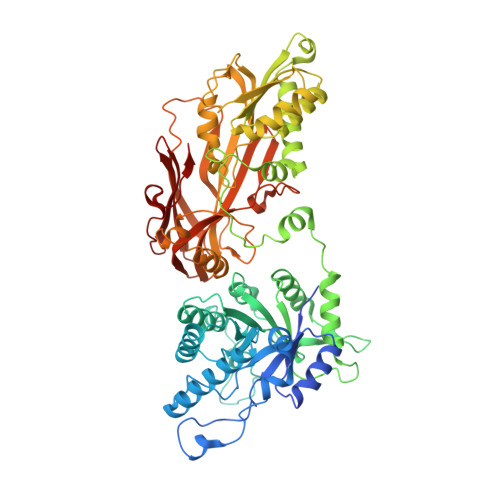 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O14744 (Homo sapiens) Explore O14744 Go to UniProtKB: O14744 | |||||
PHAROS: O14744 GTEx: ENSG00000100462 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O14744 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Methylosome protein 50 | 348 | Homo sapiens | Mutation(s): 0 Gene Names: WDR77, MEP50, WD45, HKMT1069, Nbla10071 | 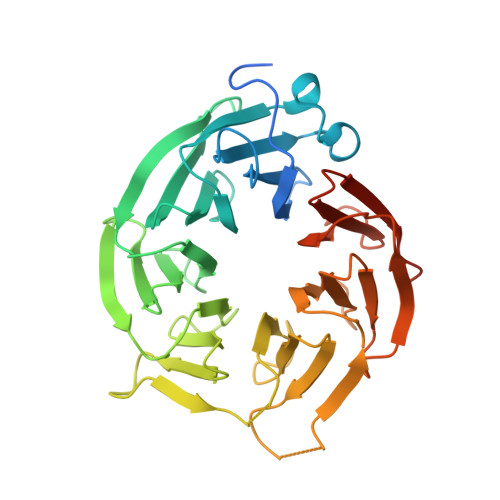 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9BQA1 (Homo sapiens) Explore Q9BQA1 Go to UniProtKB: Q9BQA1 | |||||
PHAROS: Q9BQA1 GTEx: ENSG00000116455 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9BQA1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| K8N (Subject of Investigation/LOI) Query on K8N | C [auth A] | (1~{S},2~{R},3~{S},5~{R})-3-[2-(2-azanyl-3-bromanyl-quinolin-7-yl)ethyl]-5-(4-azanylpyrrolo[2,3-d]pyrimidin-7-yl)cyclop
entane-1,2-diol C22 H23 Br N6 O2 DBSMLQTUDJVICQ-CJODITQLSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 101.132 | α = 90 |
| b = 137.854 | β = 90 |
| c = 178.617 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| xia2 | data reduction |
| Aimless | data scaling |
| PHASER | phasing |