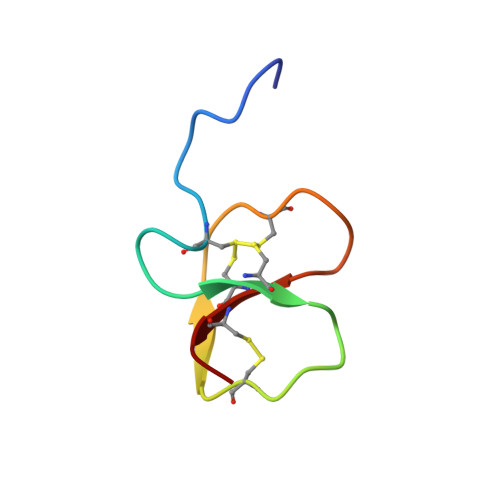
Structure, function, and evolution ofGga-AvBD11, the archetype of the structural avian-double-beta-defensin family.
Guyot, N., Meudal, H., Trapp, S., Iochmann, S., Silvestre, A., Jousset, G., Labas, V., Reverdiau, P., Loth, K., Herve, V., Aucagne, V., Delmas, A.F., Rehault-Godbert, S., Landon, C.(2020) Proc Natl Acad Sci U S A 117: 337-345
- PubMed: 31871151
- DOI: https://doi.org/10.1073/pnas.1912941117
- Primary Citation of Related Structures:
6QES, 6QET, 6QEU - PubMed Abstract:
Out of the 14 avian β-defensins identified in the Gallus gallus genome, only 3 are present in the chicken egg, including the egg-specific avian β-defensin 11 ( Gga -AvBD11). Given its specific localization and its established antibacterial activity, Gga -AvBD11 appears to play a protective role in embryonic development. Gga -AvBD11 is an atypical double-sized defensin, predicted to possess 2 motifs related to β-defensins and 6 disulfide bridges. The 3-dimensional NMR structure of the purified Gga- AvBD11 is a compact fold composed of 2 packed β-defensin domains. This fold is the archetype of a structural family, dubbed herein as avian-double-β-defensins (Av-DBD). We speculate that AvBD11 emanated from a monodomain gene ancestor and that similar events might have occurred in arthropods, leading to another structural family of less compact DBDs. We show that Gga -AvBD11 displays antimicrobial activities against gram-positive and gram-negative bacterial pathogens, the avian protozoan Eimeria tenella , and avian influenza virus. Gga -AvBD11 also shows cytotoxic and antiinvasive activities, suggesting that it may not only be involved in innate protection of the chicken embryo, but also in the (re)modeling of embryonic tissues. Finally, the contribution of either of the 2 Gga -AvBD11 domains to these biological activities was assessed, using chemically synthesized peptides. Our results point to a critical importance of the cationic N-terminal domain in mediating antibacterial, antiparasitic, and antiinvasive activities, with the C-terminal domain potentiating the 2 latter activities. Strikingly, antiviral activity in infected chicken cells, accompanied by marked cytotoxicity, requires the full-length protein.
Organizational Affiliation:
Biologie des Oiseaux et Aviculture, Institut National de la Recherche Agronomique, Université de Tours, 37380 Nouzilly, France.