Dual Inhibition of TAF1 and BET Bromodomains from the BI-2536 Kinase Inhibitor Scaffold.
Remillard, D., Buckley, D.L., Seo, H.S., Ferguson, F.M., Dhe-Paganon, S., Bradner, J.E., Gray, N.S.(2019) ACS Med Chem Lett 10: 1443-1449
- PubMed: 31620231
- DOI: https://doi.org/10.1021/acsmedchemlett.9b00243
- Primary Citation of Related Structures:
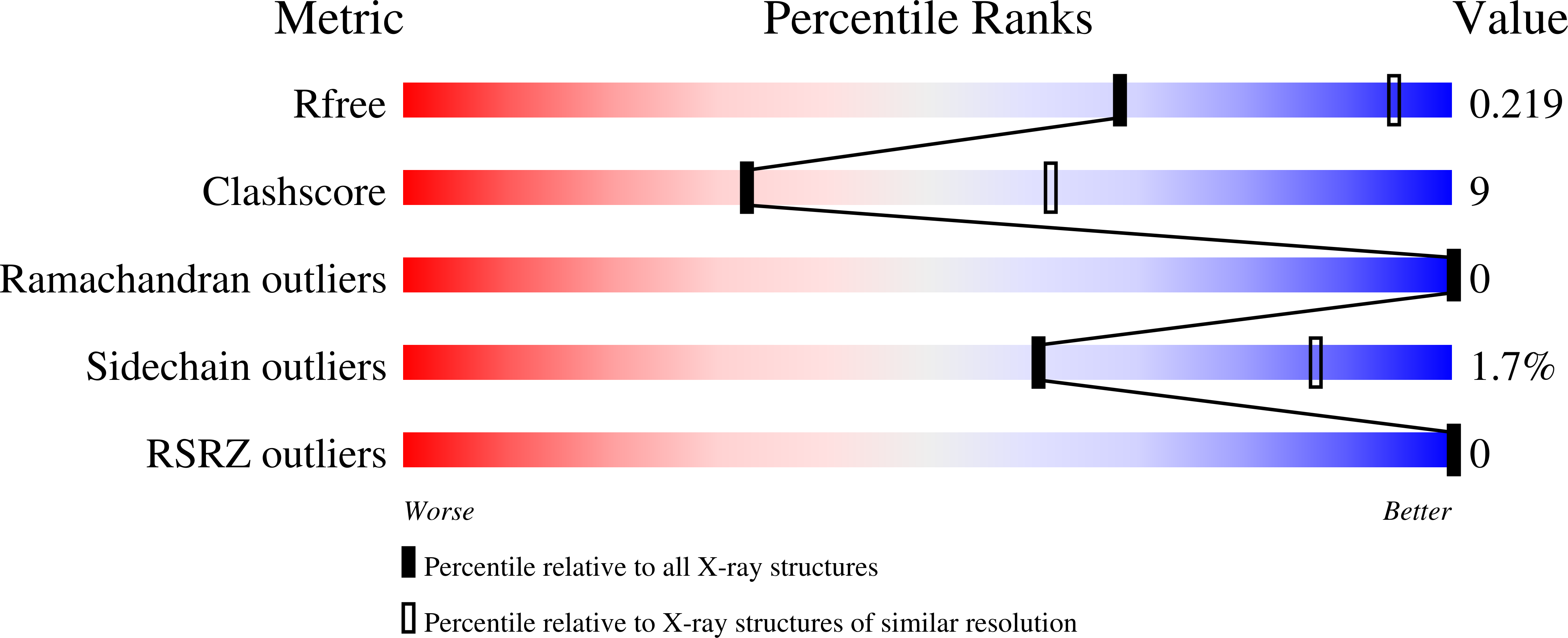
6P38 - PubMed Abstract:
Recent reports have highlighted the dual bromodomains of TAF1 (TAF1(1,2)) as synergistic with BET inhibition in cellular cancer models, engendering interest in TAF/BET polypharmacology. Here, we examine structure activity relationships within the BI-2536 PLK1 kinase inhibitor scaffold, previously reported to bind BRD4. We examine binding by this ligand to TAF1(2) and apply structure guided design strategies to discriminate binding to both the PLK1 kinase and BRD4(1) bromodomain while retaining activity on TAF1(2). Through this effort we discover potent dual inhibitors of TAF1(2)/BRD4(1), as well as biased derivatives showing marked TAF1 selectivity. We resolve X-ray crystallographic data sets to examine the mechanisms of the observed TAF1 selectivity and to provide a resource for further development of this scaffold.
Organizational Affiliation:
Department of Cancer Biology, Dana Farber Cancer Institute, Boston Massachusetts 02115, United States.