Structures of teixobactin-producing nonribosomal peptide synthetase condensation and adenylation domains.
Tan, K., Zhou, M., Jedrzejczak, R.P., Wu, R., Higuera, R.A., Borek, D., Babnigg, G., Joachimiak, A.(2020) Curr Res Struct Biol 2: 14-24
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2020) Curr Res Struct Biol 2: 14-24
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Txo1 | 873 | Eleftheria terrae | Mutation(s): 0 EC: 2 | 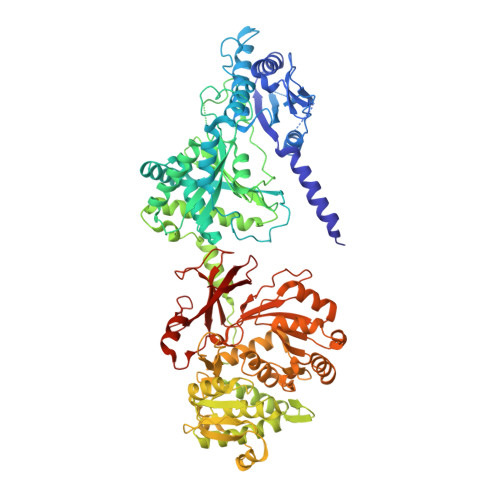 | |
UniProt | |||||
Find proteins for A0A0B5GUD2 (Eleftheria terrae) Explore A0A0B5GUD2 Go to UniProtKB: A0A0B5GUD2 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A0B5GUD2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EPE Query on EPE | P [auth A] | 4-(2-HYDROXYETHYL)-1-PIPERAZINE ETHANESULFONIC ACID C8 H18 N2 O4 S JKMHFZQWWAIEOD-UHFFFAOYSA-N |  | ||
| MES Query on MES | Q [auth A] | 2-(N-MORPHOLINO)-ETHANESULFONIC ACID C6 H13 N O4 S SXGZJKUKBWWHRA-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | C [auth A] D [auth A] E [auth A] F [auth A] G [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | R [auth A], S [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| FMT Query on FMT | B [auth A] | FORMIC ACID C H2 O2 BDAGIHXWWSANSR-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 152.965 | α = 90 |
| b = 90.749 | β = 105.99 |
| c = 98.218 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| HKL-3000 | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | HHSN272201700060C |