Ca2+-regulated Ca2+channels with an RCK gating ring control plant symbiotic associations.
Kim, S., Zeng, W., Bernard, S., Liao, J., Venkateshwaran, M., Ane, J.M., Jiang, Y.(2019) Nat Commun 10: 3703-3703
- PubMed: 31420535
- DOI: https://doi.org/10.1038/s41467-019-11698-5
- Primary Citation of Related Structures:
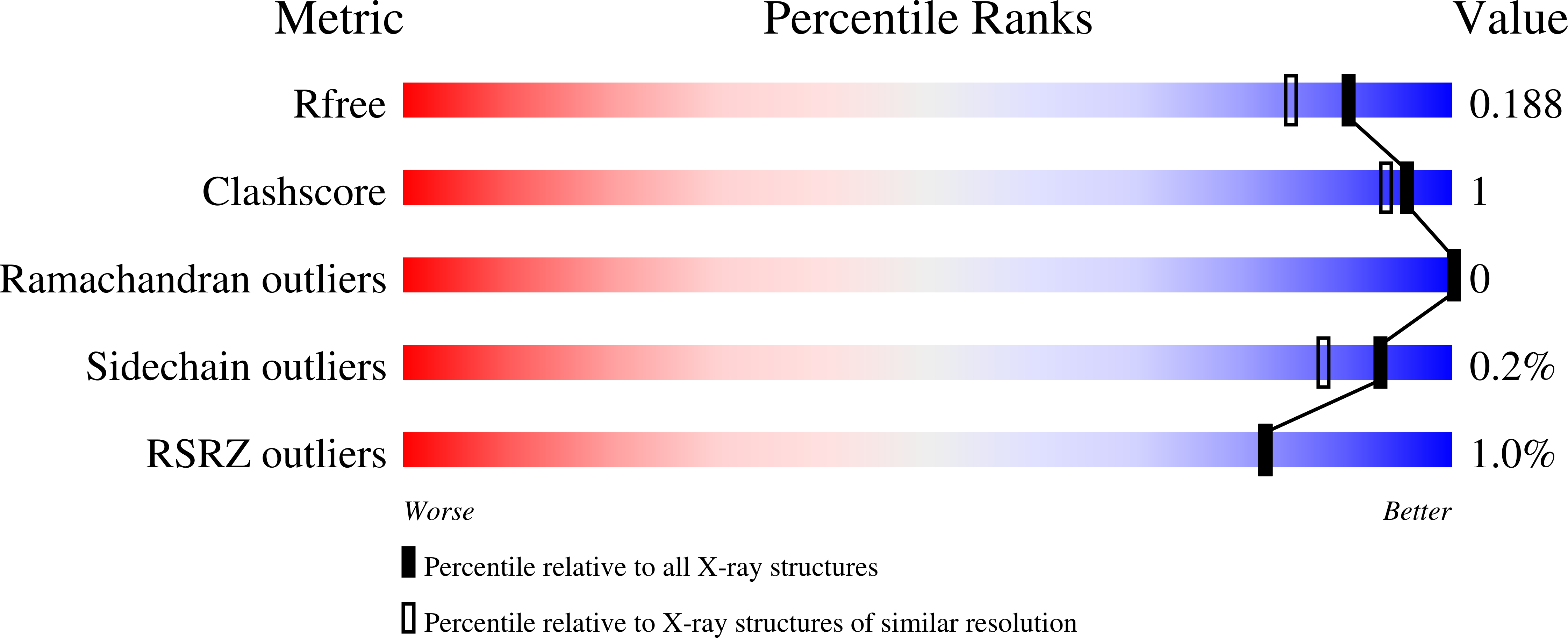
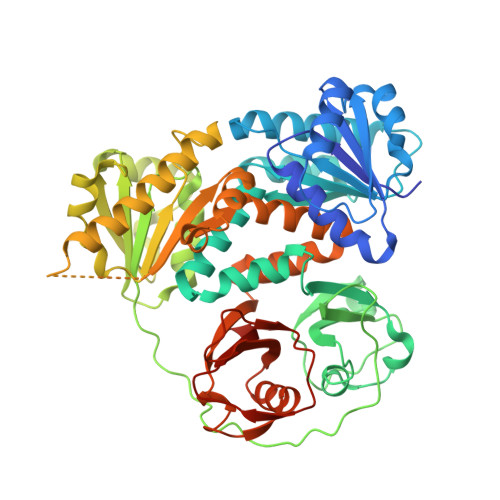
6O6J, 6O7A, 6O7C - PubMed Abstract:
A family of plant nuclear ion channels, including DMI1 (Does not Make Infections 1) and its homologs CASTOR and POLLUX, are required for the establishment of legume-microbe symbioses by generating nuclear and perinuclear Ca 2+ spiking. Here we show that CASTOR from Lotus japonicus is a highly selective Ca 2+ channel whose activation requires cytosolic/nucleosolic Ca 2+ , contrary to the previous suggestion of it being a K + channel. Structurally, the cytosolic/nucleosolic ligand-binding soluble region of CASTOR contains two tandem RCK (Regulator of Conductance for K + ) domains, and four subunits assemble into the gating ring architecture, similar to that of large conductance, Ca 2+ -gated K + (BK) channels despite the lack of sequence similarity. Multiple ion binding sites are clustered at two locations within each subunit, and three of them are identified to be Ca 2+ sites. Our in vitro and in vivo assays also demonstrate the importance of these gating-ring Ca 2+ binding sites to the physiological function of CASTOR as well as DMI1.
Organizational Affiliation:
Howard Hughes Medical Institute and Department of Physiology, University of Texas Southwestern Medical Center, Dallas, TX, USA.