Comparative structural and thermal stability studies of Cuc m 2.0101, Art v 4.0101 and other allergenic profilins.
Kapingidza, A.B., Pye, S.E., Hyduke, N., Dolamore, C., Pote, S., Schlachter, C.R., Commins, S.P., Kowal, K., Chruszcz, M.(2019) Mol Immunol 114: 19-29
- PubMed: 31326654
- DOI: https://doi.org/10.1016/j.molimm.2019.07.004
- Primary Citation of Related Structures:
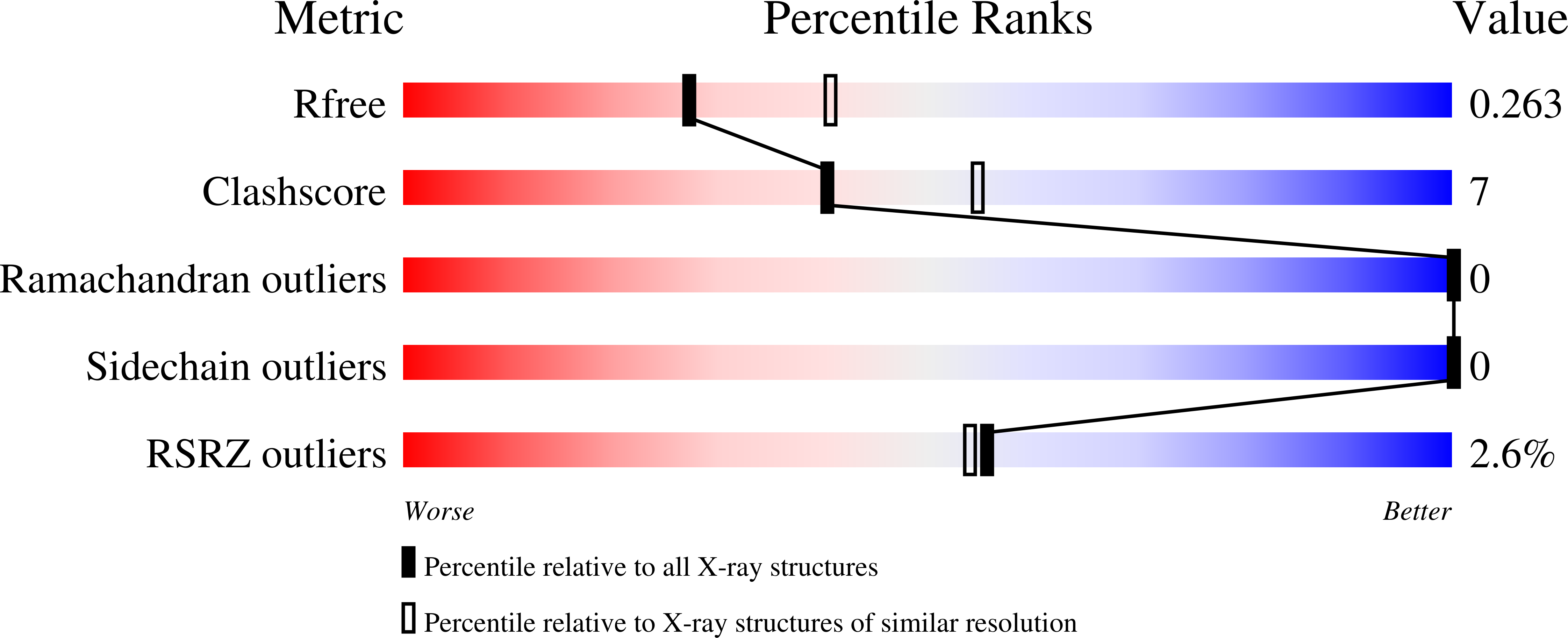
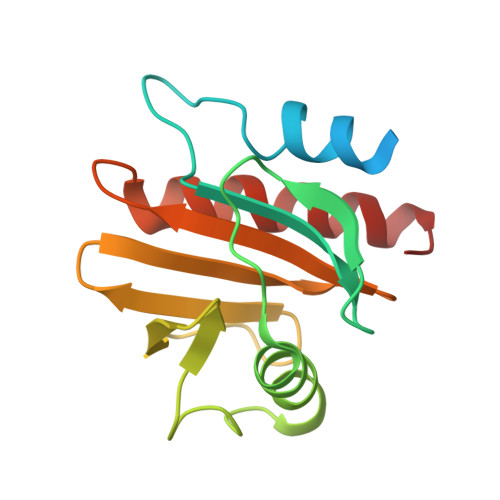
6B6J, 6MBX - PubMed Abstract:
Worldwide, more than one-third of the population suffers from allergies. A significant fraction of officially registered allergens originate from the profilin family of proteins. Profilins are small ubiquitous proteins which are found in plants, viruses and various eukaryotes including mammals. Although they are primarily regarded as minor allergens, profilins are important players in immunoglobulin E (IgE) cross-reactivity. However, in some populations profilins are recognized by IgE from at least 50% of patients allergic to a given allergen source. Cuc m 2.0101 is recognized by IgE in more than 80% of muskmelon-allergic patients. The recombinant isoallergen Cuc m 2.0101 was produced in significant quantities and its X-ray crystal structure was determined. In addition, a new Art v 4.0101 (mugwort profilin) structure was determined. The profilins Cuc m 2.0101 and Art v 4.0101 were compared in terms of their structure and thermal stability. Furthermore, structural similarities and IgE cross-reactivity between profilins from different sources are discussed to explain the molecular basis of various clinical syndromes involving this group of allergens. Special emphasis is placed on discussion of profilins' quaternary structures and their relation to biological function, as well as to protein allergenicity. Moreover, a potential impact of protein purification protocols on the structure of profilins is highlighted.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of South Carolina, Columbia, SC, 29208, United States.