Structure-Based Modification of an Anti-neuraminidase Human Antibody Restores Protection Efficacy against the Drifted Influenza Virus.
Jiang, H., Peng, W., Qi, J., Chai, Y., Song, H., Bi, Y., Rijal, P., Wang, H., Oladejo, B.O., Liu, J., Shi, Y., Gao, G.F., Townsend, A.R., Wu, Y.(2020) mBio 11
- PubMed: 33024040
- DOI: https://doi.org/10.1128/mBio.02315-20
- Primary Citation of Related Structures:
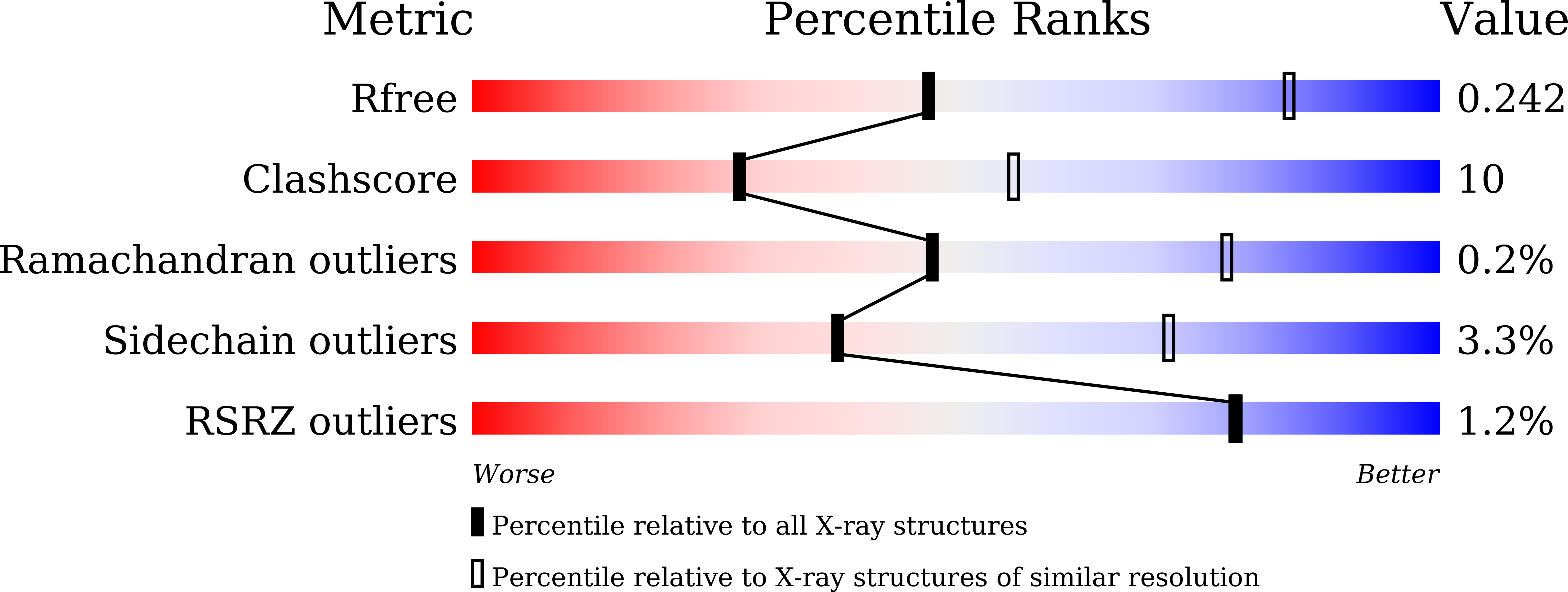
6LXI, 6LXJ, 6LXK - PubMed Abstract:
Here, we investigate a monoclonal antibody, Z2B3, isolated from an H7N9-infected patient, that exhibited cross-reactivity to both N9 (group 2) and a broad range of seasonal and avian N1 (group 1) proteins but lost activity to the N1 with the substitution K432E. This substitution exists in 99.25% of seasonal influenza strains after 2013. The NA-Z2B3 complex structures indicated that Z2B3 binds within the conserved active site of the neuraminidase (NA) protein. A salt bridge between D102 in Z2B3 and K432 in NA plays an important role in binding. Structure-based modification of Z2B3 with D102R in heavy chain reversed the salt bridge and restored the binding and inhibition of N1 with E432. Furthermore, Z2B3-D102R can protect mice from A/Serbia/NS-601/2014 H1N1 virus (NA contains E432) infection while the wild-type Z2B3 antibody shows no protection. This study demonstrates that a broadly reactive and protective antibody to NA can be in principle edited to restore binding and inhibition to recently drifted N1 NA and regain protection against the variant influenza strain. IMPORTANCE The immune system produces antibodies to protect the human body from harmful invaders. The monoclonal antibody (MAb) is one kind of effective antivirals. In this study, we isolated an antibody (Z2B3) from an H7N9 influenza virus-infected child. It shows cross-reactivity to both group 1 (N1) and group 2 (N9) neuraminidases (NAs) but is sensitive to N1 NA with a K432E substitution. Structural analysis of the NA-antibody fragment antigen-binding (Fab) complex provides a clue for antibody modification, and the modified antibody restored binding and inhibition to recently drifted N1 NA and regained protection against the variant influenza strain. This finding suggests that antibodies to NA may be a useful therapy and can be in principle edited to defeat drifted influenza virus.
Organizational Affiliation:
College of Veterinary Medicine, China Agricultural University, Beijing, China.