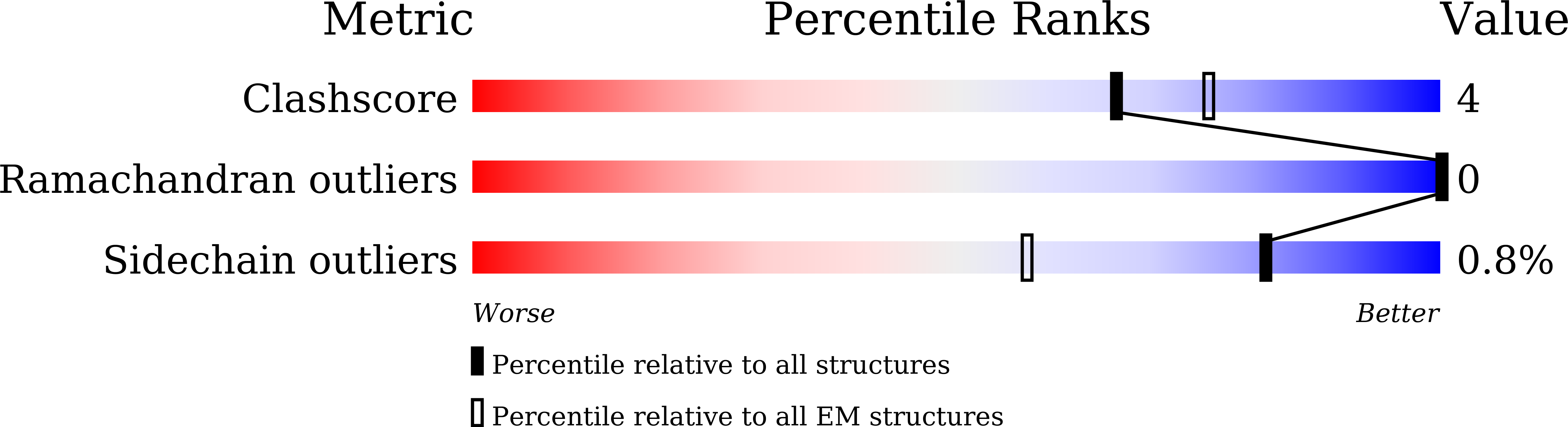Viral neutralization by antibody-imposed physical disruption.
Zheng, Q., Jiang, J., He, M., Zheng, Z., Yu, H., Li, T., Xue, W., Tang, Z., Ying, D., Li, Z., Song, S., Liu, X., Wang, K., Zhang, Z., Wang, D., Wang, Y., Yan, X., Zhao, Q., Zhang, J., Gu, Y., Li, S., Xia, N.(2019) Proc Natl Acad Sci U S A
- PubMed: 31818956
- DOI: https://doi.org/10.1073/pnas.1916028116
- Primary Citation of Related Structures:
6LAT, 6LB0 - PubMed Abstract:
In adaptive immunity, organisms produce neutralizing antibodies (nAbs) to eliminate invading pathogens. Here, we explored whether viral neutralization could be attained through the physical disruption of a virus upon nAb binding. We report the neutralization mechanism of a potent nAb 8C11 against the hepatitis E virus (HEV), a nonenveloped positive-sense single-stranded RNA virus associated with abundant acute hepatitis. The 8C11 binding flanks the protrusion spike of the HEV viruslike particles (VLPs) and leads to tremendous physical collision between the antibody and the capsid, dissociating the VLPs into homodimer species within 2 h. Cryo-electron microscopy reconstruction of the dissociation intermediates at an earlier (15-min) stage revealed smeared protrusion spikes and a loss of icosahedral symmetry with the capsid core remaining unchanged. This structural disruption leads to the presence of only a few native HEV virions in the ultracentrifugation pellet and exposes the viral genome. Conceptually, we propose a strategy to raise collision-inducing nAbs against single spike moieties that feature in the context of the entire pathogen at positions where the neighboring space cannot afford to accommodate an antibody. This rationale may facilitate unique vaccine development and antimicrobial antibody design.
Organizational Affiliation:
State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Life Sciences, School of Public Health, Xiamen University, 361102 Xiamen, China.