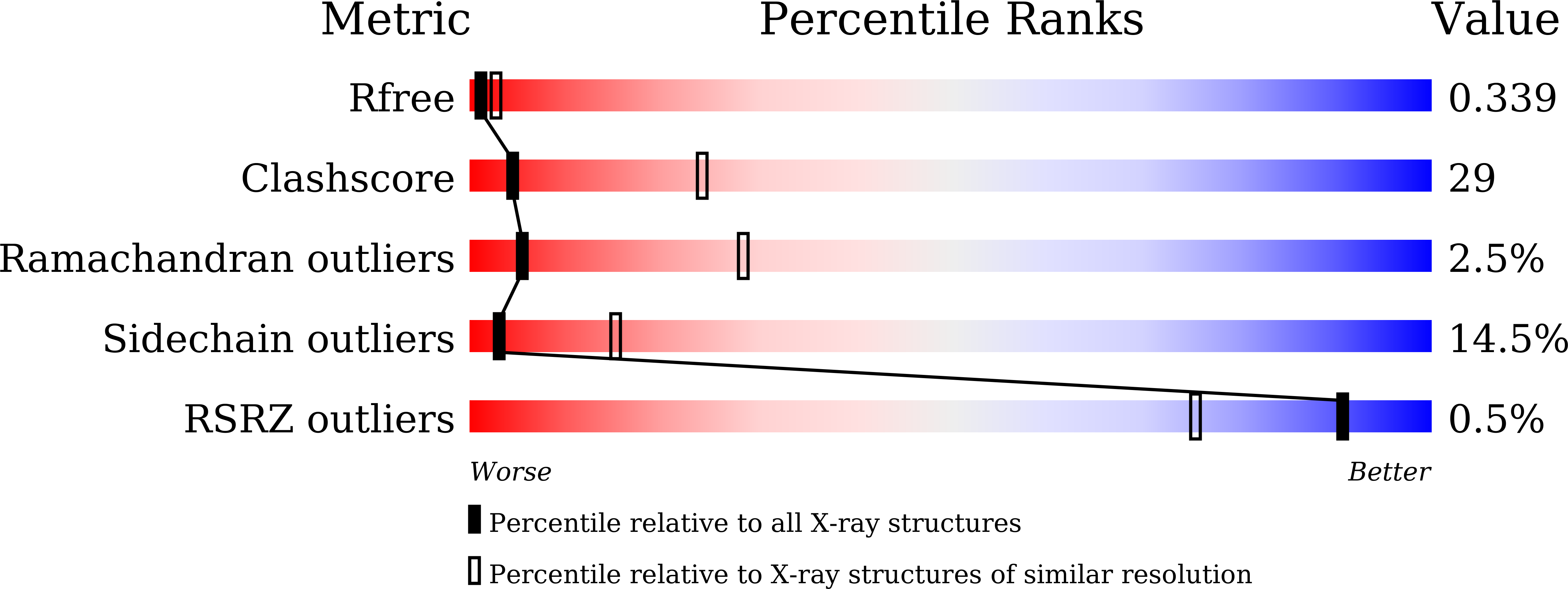
Structural insights into CYP107G1 from rapamycin-producing Streptomyces rapamycinicus.
Kim, V., Lim, Y.R., Lee, I., Lee, J.H., Han, S., Pham, T.V., Kim, H., Lee, R., Kang, L.W., Kim, D.(2020) Arch Biochem Biophys 692: 108544-108544
- PubMed: 32822639
- DOI: https://doi.org/10.1016/j.abb.2020.108544
- Primary Citation of Related Structures:
6L39, 6L3A - PubMed Abstract:
Rapamycin is a clinically important macrolide agent with immunosuppressant and antiproliferative properties, produced by the actinobacterium, Streptomyces rapamycinicus. Two cytochrome P450 enzymes are involved in the biosynthesis of rapamycin. CYP107G1 and CYP122A2 catalyze the oxidation reactions of C27 and C9 of pre-rapamycin, respectively. To understand the structural and biochemical features of P450 enzymes in rapamycin biosynthesis, the CYP107G1 and CYP122A2 genes were cloned, their recombinant proteins were expressed in Escherichia coli, and the purified enzymes were characterized. Both enzymes displayed low spin states in the absolute spectra of ferric forms, and the titrations with rapamycin induced type I spectral changes with K d values of 4.4 ± 0.4 and 3.0 ± 0.3 μM for CYP107G1 and CYP122A2, respectively. The X-ray crystal structures of CYP107G1 and its co-crystal complex with everolimus, a clinical rapamycin derivative, were determined at resolutions of 2.9 and 3.0 Å, respectively. The overall structure of CYP107G1 adopts the canonical scaffold of cytochrome P450 and possesses large substrate pocket. The distal face of the heme group is exposed to solvents to accommodate macrolide access. When the structure of the everolimus-bound CYP107G1 complex (CYP107G1-Eve) was compared to that of the ligand-free CYP107G1 form, no significant conformational change was observed. Hence, CYP107G1 has a relatively rigid structure with versatile loops to accommodate a bulky substrate. The everolimus molecule is bound to the substrate-binding pocket in the shape of a squeezed donut, and its elongated structure is bound perpendicular to a planar heme plane and I-helix.
Organizational Affiliation:
Department of Biological Sciences, Konkuk University, Seoul, 05025, Republic of Korea.