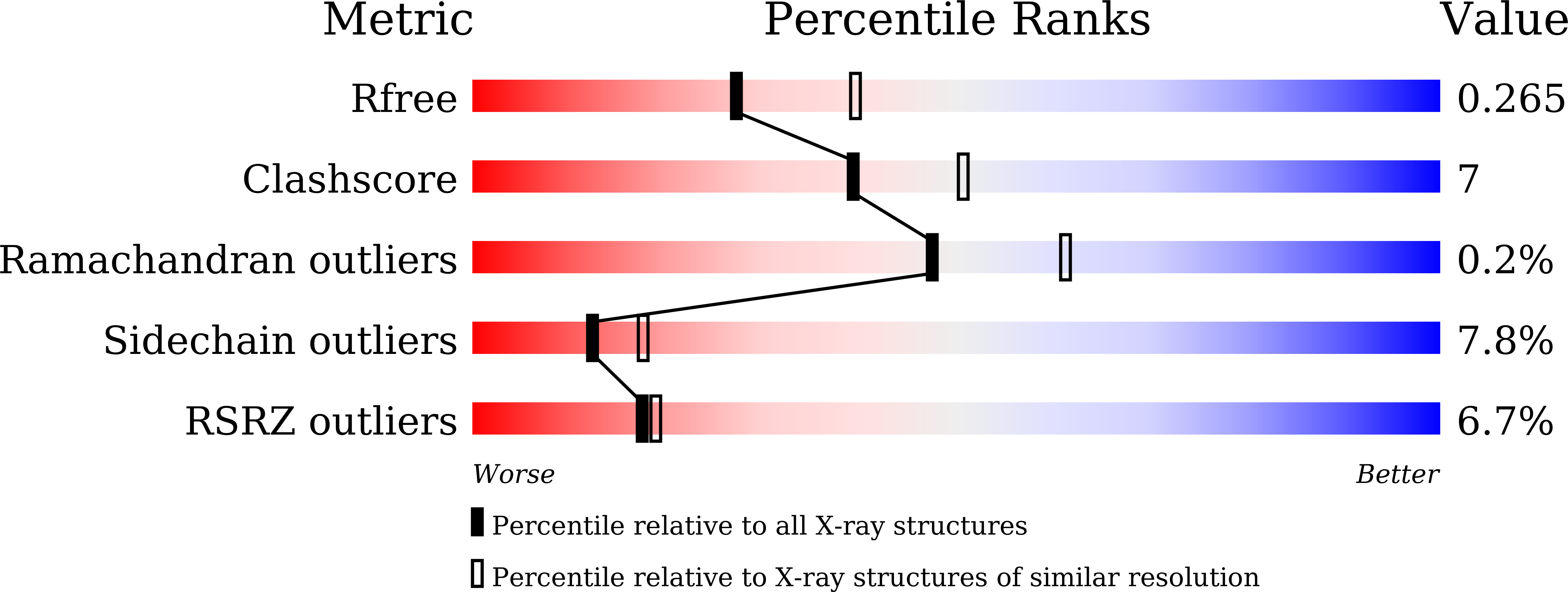
Distinct molecular assembly of homologous peroxiredoxins from Pyrococcus horikoshii and Thermococcus kodakaraensis.
Himiyama, T., Oshima, M., Uegaki, K., Nakamura, T.(2019) J Biochem 166: 89-95
- PubMed: 30796432
- DOI: https://doi.org/10.1093/jb/mvz013
- Primary Citation of Related Structures:
6ITZ, 6IU0, 6IU1 - PubMed Abstract:
Peroxiredoxins from Pyrococcus horikoshii (PhPrx) and Thermococcus kodakaraensis (TkPrx) are highly homologous proteins sharing 196 of the 216 residues. We previously reported a pentagonal ring-type decameric structure of PhPrx. Here, we present the crystal structure of TkPrx. Despite their homology, unlike PhPrx, the quaternary structure of TkPrx was found to be a dodecamer comprised of six homodimers arranged in a hexagonal ring-type assembly. The possibility of the redox-dependent conversion of the molecular assembly, which had been observed in PhPrx, was excluded for TkPrx based on the crystal structure of a mutant in which all of the cysteine residues were substituted with serine. The monomer structures of the dodecameric TkPrx and decameric PhPrx coincided well, but there was a slight difference in the relative orientation of the two domains. Molecular assembly of PhPrx and TkPrx in solution evaluated by gel-filtration chromatography was consistent with the crystallographic results. For both PhPrx and TkPrx, the gel-filtration elution volume slightly increased with a decrease in the protein concentration, suggesting the existence of an equilibrium state between the decameric/dodecameric ring and lower-order assembly. This structural assembly difference between highly homologous Prxs suggests a significant influence of quaternary structure on function, worthy of further exploration.
Organizational Affiliation:
National Institute of Advanced Industrial Science and Technology, 1-8-31 Midorigaoka, Ikeda, Osaka, Japan.