A mechanism-based GlcNAc-inspired cyclophellitol inactivator of the peptidoglycan recycling enzyme NagZ reverses resistance to beta-lactams in Pseudomonas aeruginosa.
Ho, L.A., Winogrodzki, J.L., Debowski, A.W., Madden, Z., Vocadlo, D.J., Mark, B.L., Stubbs, K.A.(2018) Chem Commun (Camb) 54: 10630-10633
- PubMed: 30178799
- DOI: https://doi.org/10.1039/c8cc05281f
- Primary Citation of Related Structures:
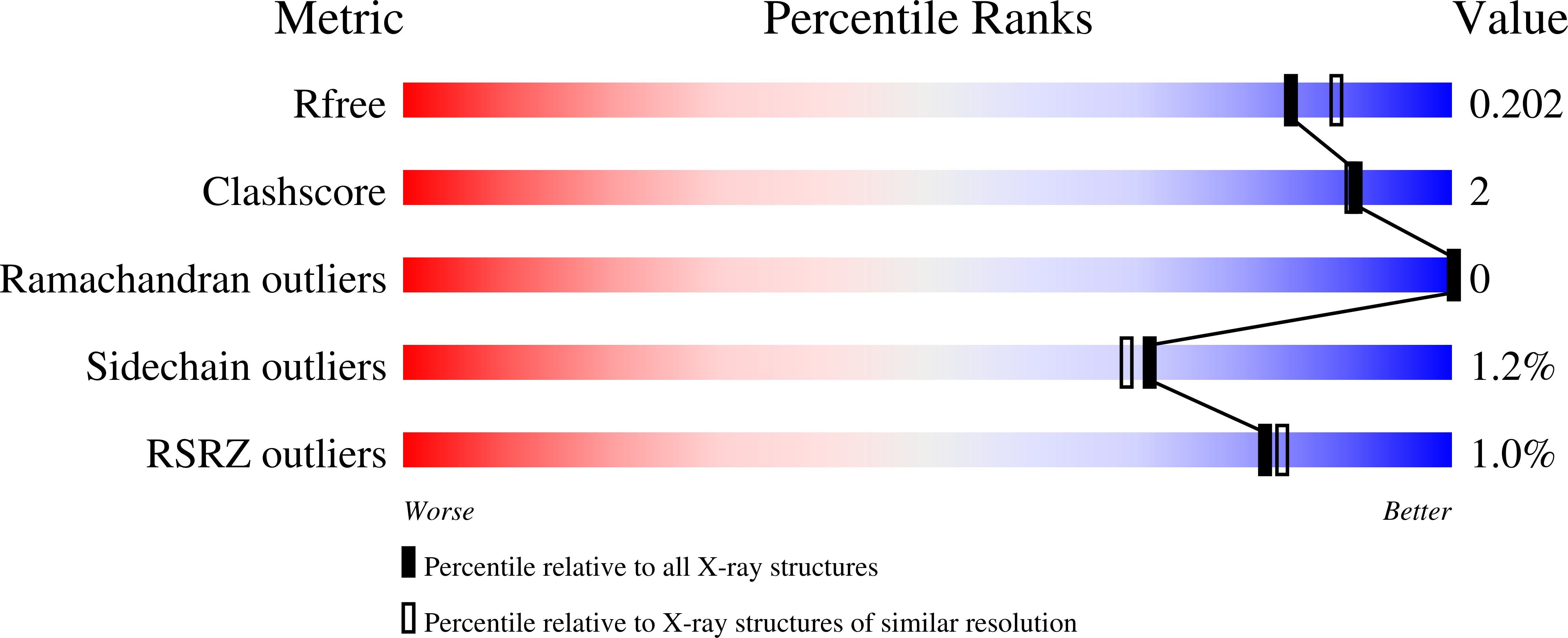
6DTE - PubMed Abstract:
The development of a potent mechanism-based inactivator of NagZ, an enzyme critical to the production of inducible AmpC β-lactamase in Gram-negative bacteria, is presented. This inactivator significantly reduces MIC values for important β-lactams against a clinically relevant strain of Pseudomonas aeruginosa.
Organizational Affiliation:
School of Molecular Sciences, University of Western Australia, Crawley, WA 6009, Australia. keith.stubbs@uwa.edu.au.