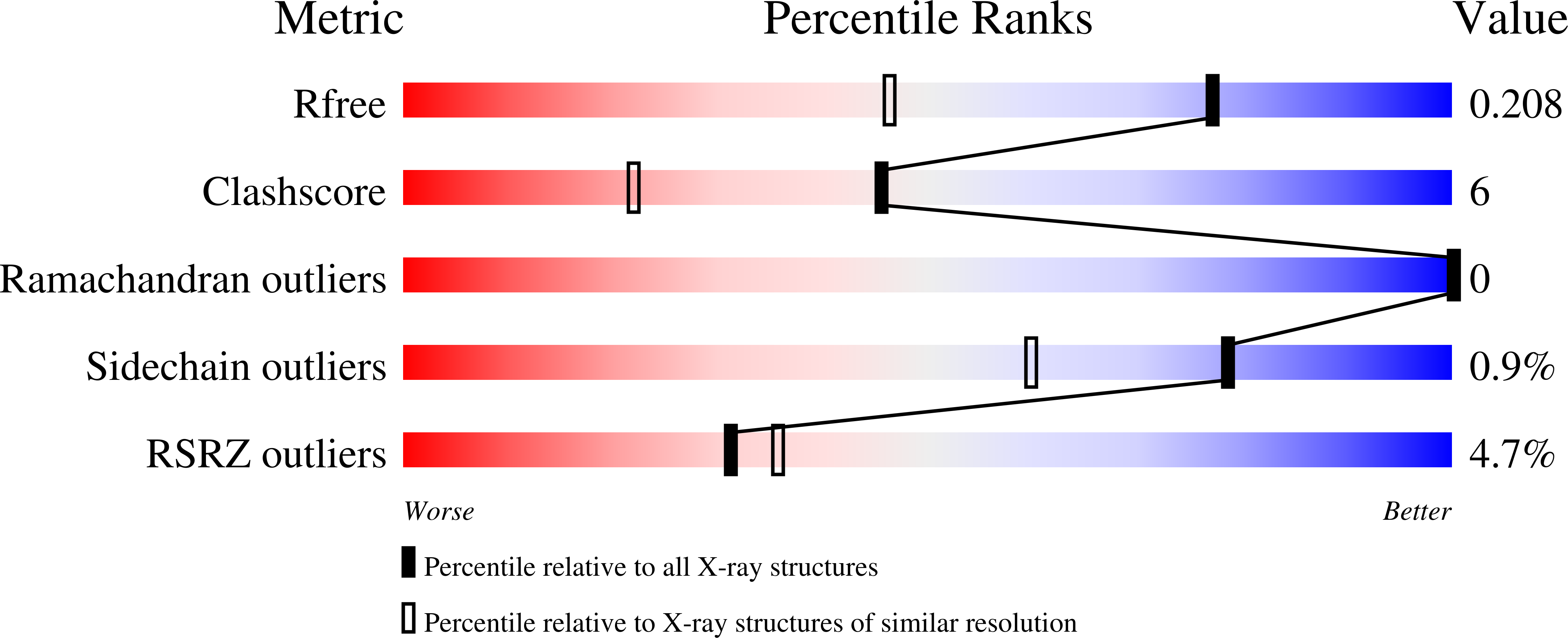
Plazomicin Retains Antibiotic Activity against Most Aminoglycoside Modifying Enzymes.
Cox, G., Ejim, L., Stogios, P.J., Koteva, K., Bordeleau, E., Evdokimova, E., Sieron, A.O., Savchenko, A., Serio, A.W., Krause, K.M., Wright, G.D.(2018) ACS Infect Dis 4: 980-987
- PubMed: 29634241
- DOI: https://doi.org/10.1021/acsinfecdis.8b00001
- Primary Citation of Related Structures:
5US1, 6CD7 - PubMed Abstract:
Plazomicin is a next-generation, semisynthetic aminoglycoside antibiotic currently under development for the treatment of infections due to multidrug-resistant Enterobacteriaceae. The compound was designed by chemical modification of the natural product sisomicin to provide protection from common aminoglycoside modifying enzymes that chemically alter these drugs via N-acetylation, O-adenylylation, or O-phosphorylation. In this study, plazomicin was profiled against a panel of isogenic strains of Escherichia coli individually expressing twenty-one aminoglycoside resistance enzymes. Plazomicin retained antibacterial activity against 15 of the 17 modifying enzyme-expressing strains tested. Expression of only two of the modifying enzymes, aac(2')-Ia and aph(2″)-IVa, decreased plazomicin potency. On the other hand, expression of 16S rRNA ribosomal methyltransferases results in a complete lack of plazomicin potency. In vitro enzymatic assessment confirmed that AAC(2')-Ia and APH(2'')-IVa (aminoglycoside acetyltransferase, AAC; aminoglycoside phosphotransferase, APH) were able to utilize plazomicin as a substrate. AAC(2')-Ia and APH(2'')-IVa are limited in their distribution to Providencia stuartii and Enterococci, respectively. These data demonstrate that plazomicin is not modified by a broad spectrum of common aminoglycoside modifying enzymes including those commonly found in Enterobacteriaceae. However, plazomicin is inactive in the presence of 16S rRNA ribosomal methyltransferases, which should be monitored in future surveillance programs.
Organizational Affiliation:
M.G. DeGroote Institute for Infectious Disease Research, Department of Biochemistry and Biomedical Sciences, DeGroote School of Medicine , McMaster University , 1280 Main Street West , Hamilton , Ontario L8N 4K1 , Canada.