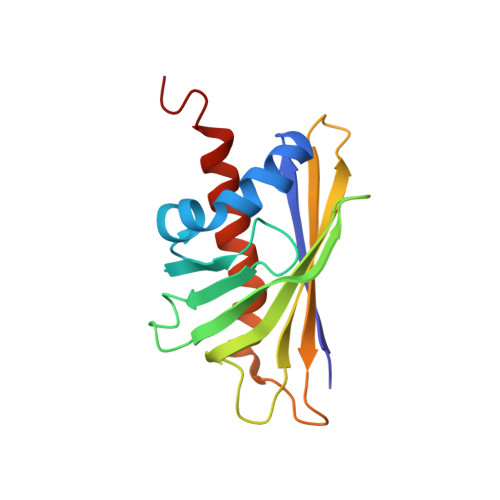
Structural basis for cross-reactivity and conformation fluctuation of the major beech pollen allergen Fag s 1.
Moraes, A.H., Asam, C., Almeida, F.C.L., Wallner, M., Ferreira, F., Valente, A.P.(2018) Sci Rep 8: 10512-10512
- PubMed: 30002383
- DOI: https://doi.org/10.1038/s41598-018-28358-1
- Primary Citation of Related Structures:
6ALK - PubMed Abstract:
Fag s 1 is a member of the Pathogen Related protein family 10 (PR-10) and can elicit cross-reaction with IgE antibodies produced against the birch pollen allergen Bet v 1. The Nuclear Magnetic Resonance (NMR) structure of Fag s 1 is presented along with its dynamic properties. It shares 66% identity with Bet v 1 and exhibits the expected three α-helices and seven β-sheets arranged as a semi-beta barrel and exposing the residues mapped as the Bet v 1 IgE epitope. The structural dynamics of Fag s 1 were monitored on the fast and intermediate timescales, using relaxation rates. The complex dynamics of Fag s 1 are closely related to the internal cavity, and they modulate IgE and ligand binding.
Organizational Affiliation:
Chemistry Department, Federal University of Minas Gerais, Belo Horizonte, Brazil.