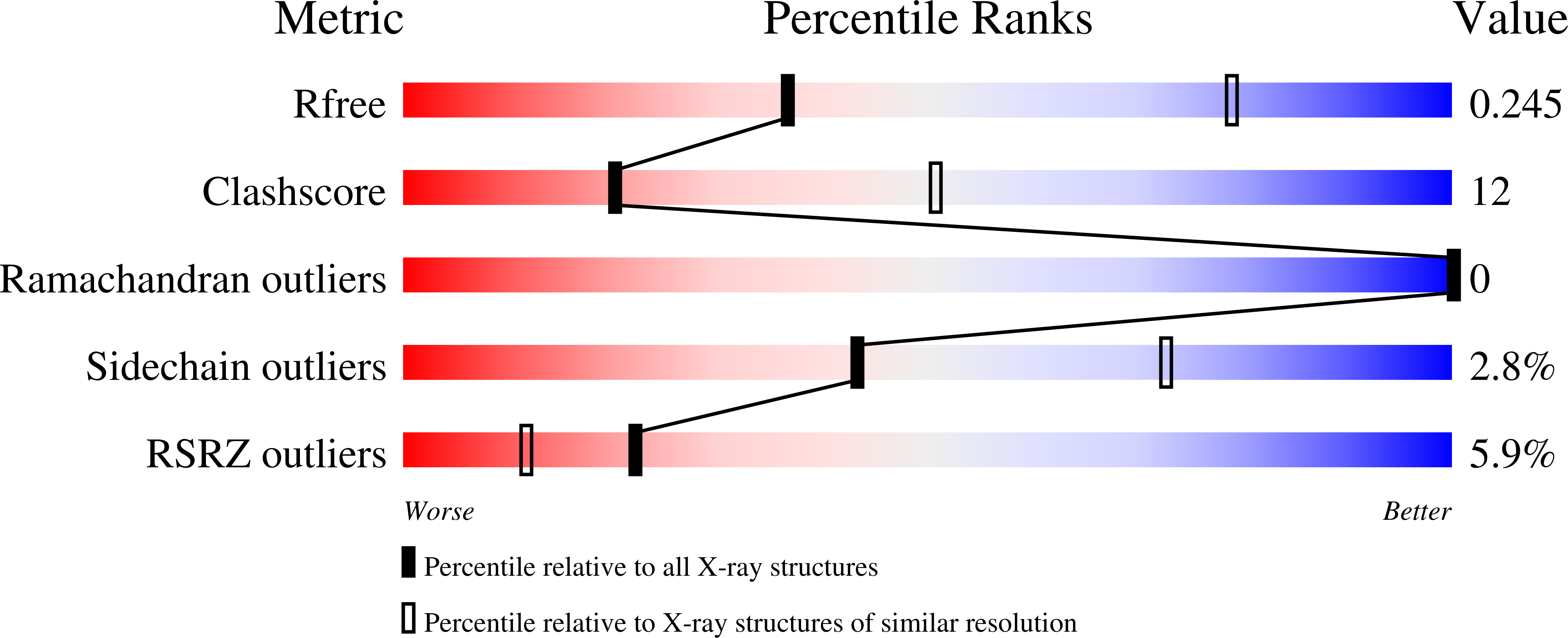
Novel quinazolinone inhibitors of the Pseudomonas aeruginosa quorum sensing transcriptional regulator PqsR.
Grossman, S., Soukarieh, F., Richardson, W., Liu, R., Mashabi, A., Emsley, J., Williams, P., Camara, M., Stocks, M.J.(2020) Eur J Med Chem 208: 112778-112778
- PubMed: 32927392
- DOI: https://doi.org/10.1016/j.ejmech.2020.112778
- Primary Citation of Related Structures:
6YZ3, 6Z07, 6Z17, 6Z5K - PubMed Abstract:
Rising numbers of cases of multidrug- and extensively drug-resistant Pseudomonas aeruginosa over recent years have created an urgent need for novel therapeutic approaches to cure potentially fatal infections. One such approach is virulence attenuation where anti-virulence compounds, designed to reduce pathogenicity without affording bactericidal effects, are employed to treat infections. P. aeruginosa uses the pqs quorum sensing (QS) system, to coordinate the expression of a large number of virulence determinants as well as bacterial-host interactions and hence represents an excellent anti-virulence target. We report the synthesis and identification of a new series of thiazole-containing quinazolinones capable of inhibiting PqsR, the transcriptional regulator of the pqs QS system. The compounds demonstrated high potency (IC 50 < 300 nM) in a whole-cell assay, using a mCTX:P pqsA -lux-based bioreporter for the P. aeruginosa PAO1-L and PA14 strains. Structural evaluation defined the binding modes of four analogues in the ligand-binding domain of PqsR through X-ray crystallography. Further work showed the ability of 6-chloro-3((2-pentylthiazol-4-yl)methyl)quinazolin-4(3H)-one (18) and 6-chloro-3((2-hexylthiazol-4-yl)methyl)quinazolin-4(3H)-one (19) to attenuate production of the PqsR-regulated virulence factor pyocyanin. Compounds 18 and 19 showed a low cytotoxic profile in the A549 human epithelial lung cell line making them suitable candidates for further pre-clinical evaluation.
Organizational Affiliation:
School of Pharmacy, University of Nottingham Biodiscovery Institute, University Park, Nottingham, Nottinghamshire, NG7 2RD, UK.