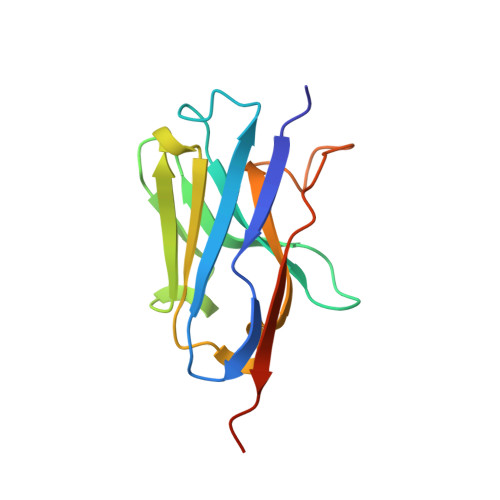
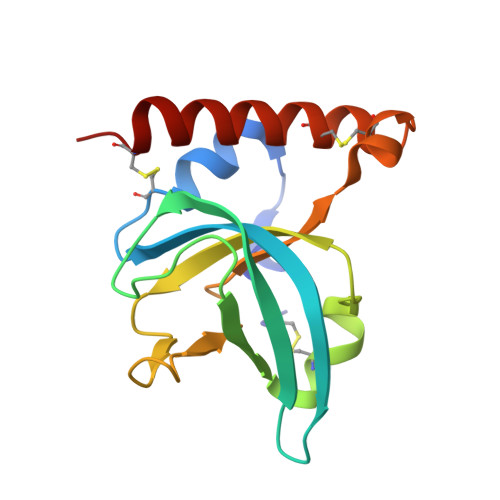
A Complement C3-Specific Nanobody for Modulation of the Alternative Cascade Identifies the C-Terminal Domain of C3b as Functional in C5 Convertase Activity.
Pedersen, H., Jensen, R.K., Jensen, J.M.B., Fox, R., Pedersen, D.V., Olesen, H.G., Hansen, A.G., Christiansen, D., Mazarakis, S.M.M., Lojek, N., Hansen, P., Gadeberg, T.A.F., Zarantonello, A., Laursen, N.S., Mollnes, T.E., Johnson, M.B., Stevens, B., Thiel, S., Andersen, G.R.(2020) J Immunol 205: 2287-2300
- PubMed: 32938727
- DOI: https://doi.org/10.4049/jimmunol.2000752
- Primary Citation of Related Structures:
6XZU - PubMed Abstract:
The complement system is an intricate cascade of the innate immune system and plays a key role in microbial defense, inflammation, organ development, and tissue regeneration. There is increasing interest in developing complement regulatory and inhibitory agents to treat complement dysfunction. In this study, we describe the nanobody hC3Nb3, which is specific for the C-terminal C345c domain of human and mouse complement component C3/C3b/C3c and potently inhibits C3 cleavage by the alternative pathway. A high-resolution structure of the hC3Nb3-C345c complex explains how the nanobody blocks proconvertase assembly. Surprisingly, although the nanobody does not affect classical pathway-mediated C3 cleavage, hC3Nb3 inhibits classical pathway-driven hemolysis, suggesting that the C-terminal domain of C3b has an important function in classical pathway C5 convertase activity. The hC3Nb3 nanobody binds C3 with low nanomolar affinity in an SDS-resistant complex, and the nanobody is demonstrated to be a powerful reagent for C3 detection in immunohistochemistry and flow cytometry. Overall, the hC3Nb3 nanobody represents a potent inhibitor of both the alternative pathway and the terminal pathway, with possible applications in complement research, diagnostics, and therapeutics.
Organizational Affiliation:
Department of Molecular Biology and Genetics, Aarhus University, DK-8000 Aarhus, Denmark.