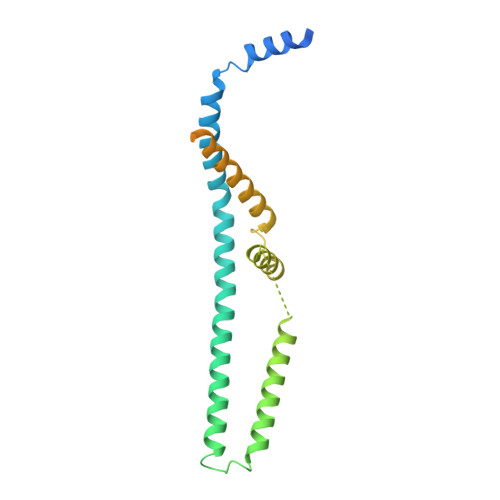
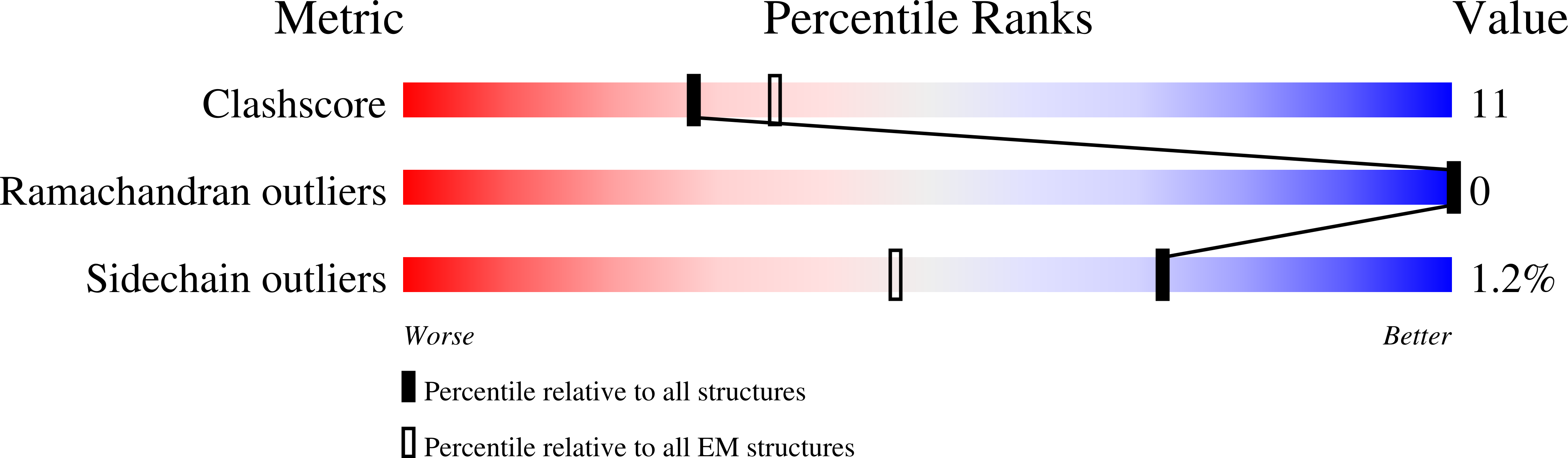Structure and Reconstitution of an MCU-EMRE Mitochondrial Ca 2+ Uniporter Complex.
Wang, C., Baradaran, R., Long, S.B.(2020) J Mol Biol 432: 5632-5648
- PubMed: 32841658
- DOI: https://doi.org/10.1016/j.jmb.2020.08.013
- Primary Citation of Related Structures:
6X4S - PubMed Abstract:
The proteins MCU and EMRE form the minimal functional unit of the mitochondrial calcium uniporter complex in metazoans, a highly selective and tightly controlled Ca 2+ channel of the inner mitochondrial membrane that regulates cellular metabolism. Here we present functional reconstitution of an MCU-EMRE complex from the red flour beetle, Tribolium castaneum, and a cryo-EM structure of the complex at 3.5 Å resolution. Using a novel assay, we demonstrate robust Ca 2+ uptake into proteoliposomes containing the purified complex. Uptake is dependent on EMRE and also on the mitochondrial lipid cardiolipin. The structure reveals a tetrameric channel with a single ion pore. EMRE is located at the periphery of the transmembrane domain and associates primarily with the first transmembrane helix of MCU. Coiled-coil and juxtamembrane domains within the matrix portion of the complex adopt markedly different conformations than in a structure of a human MCU-EMRE complex, suggesting that the structures represent different conformations of these functionally similar metazoan channels.
Organizational Affiliation:
Structural Biology Program, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.