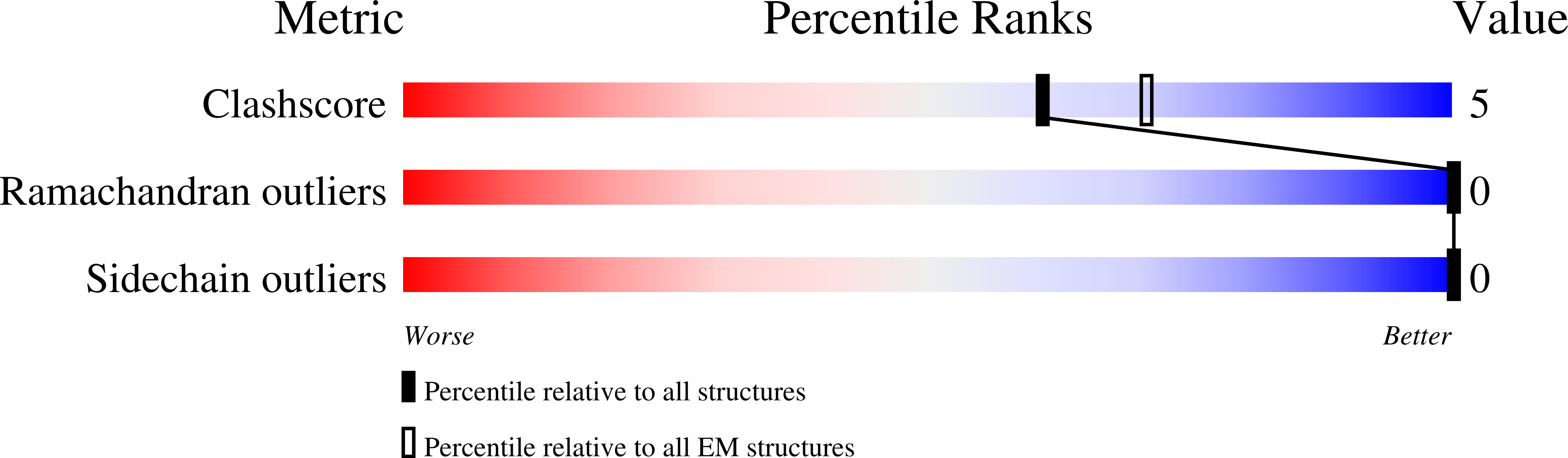Limited Dishevelled/Axin oligomerization determines efficiency of Wnt/ beta-catenin signal transduction.
Kan, W., Enos, M.D., Korkmazhan, E., Muennich, S., Chen, D.H., Gammons, M.V., Vasishtha, M., Bienz, M., Dunn, A.R., Skiniotis, G., Weis, W.I.(2020) Elife 9
- PubMed: 32297861
- DOI: https://doi.org/10.7554/eLife.55015
- Primary Citation of Related Structures:
6VCC - PubMed Abstract:
In Wnt/β-catenin signaling, the transcriptional coactivator β-catenin is regulated by its phosphorylation in a complex that includes the scaffold protein Axin and associated kinases. Wnt binding to its coreceptors activates the cytosolic effector Dishevelled (Dvl), leading to the recruitment of Axin and the inhibition of β-catenin phosphorylation. This process requires interaction of homologous DIX domains present in Dvl and Axin, but is mechanistically undefined. We show that Dvl DIX forms antiparallel, double-stranded oligomers in vitro, and that Dvl in cells forms oligomers typically <10 molecules at endogenous expression levels. Axin DIX (DAX) forms small single-stranded oligomers, but its self-association is stronger than that of DIX. DAX caps the ends of DIX oligomers, such that a DIX oligomer has at most four DAX binding sites. The relative affinities and stoichiometry of the DIX-DAX interaction provide a mechanism for efficient inhibition of β-catenin phosphorylation upon Axin recruitment to the Wnt receptor complex.
Organizational Affiliation:
Department of Structural Biology and Stanford University School of Medicine, Stanford, United States.