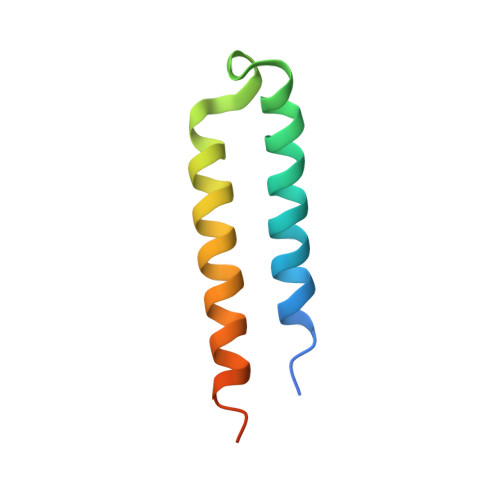
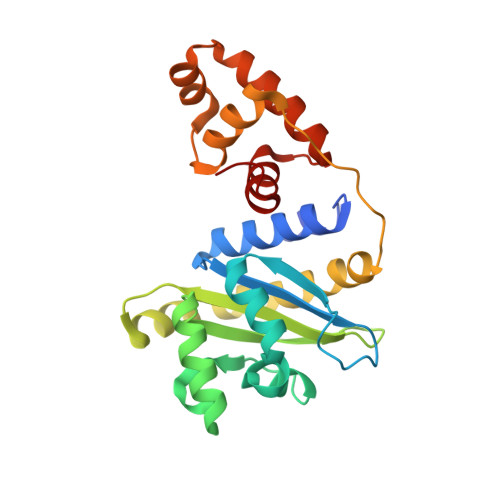
A phage-encoded anti-activator inhibits quorum sensing in Pseudomonas aeruginosa.
Shah, M., Taylor, V.L., Bona, D., Tsao, Y., Stanley, S.Y., Pimentel-Elardo, S.M., McCallum, M., Bondy-Denomy, J., Howell, P.L., Nodwell, J.R., Davidson, A.R., Moraes, T.F., Maxwell, K.L.(2021) Mol Cell 81: 571-583.e6
- PubMed: 33412111
- DOI: https://doi.org/10.1016/j.molcel.2020.12.011
- Primary Citation of Related Structures:
6V7U, 6V7V, 6V7W, 6V7X - PubMed Abstract:
The arms race between bacteria and phages has led to the evolution of diverse anti-phage defenses, several of which are controlled by quorum-sensing pathways. In this work, we characterize a quorum-sensing anti-activator protein, Aqs1, found in Pseudomonas phage DMS3. We show that Aqs1 inhibits LasR, the master regulator of quorum sensing, and present the crystal structure of the Aqs1-LasR complex. The 69-residue Aqs1 protein also inhibits PilB, the type IV pilus assembly ATPase protein, which blocks superinfection by phages that require the pilus for infection. This study highlights the remarkable ability of small phage proteins to bind multiple host proteins and disrupt key biological pathways. As quorum sensing influences various anti-phage defenses, Aqs1 provides a mechanism by which infecting phages might simultaneously dampen multiple defenses. Because quorum-sensing systems are broadly distributed across bacteria, this mechanism of phage counter-defense may play an important role in phage-host evolutionary dynamics.
Organizational Affiliation:
Department of Biochemistry, University of Toronto, MaRS West Tower, 661 University Avenue, Toronto, ON M5G 1M1, Canada.