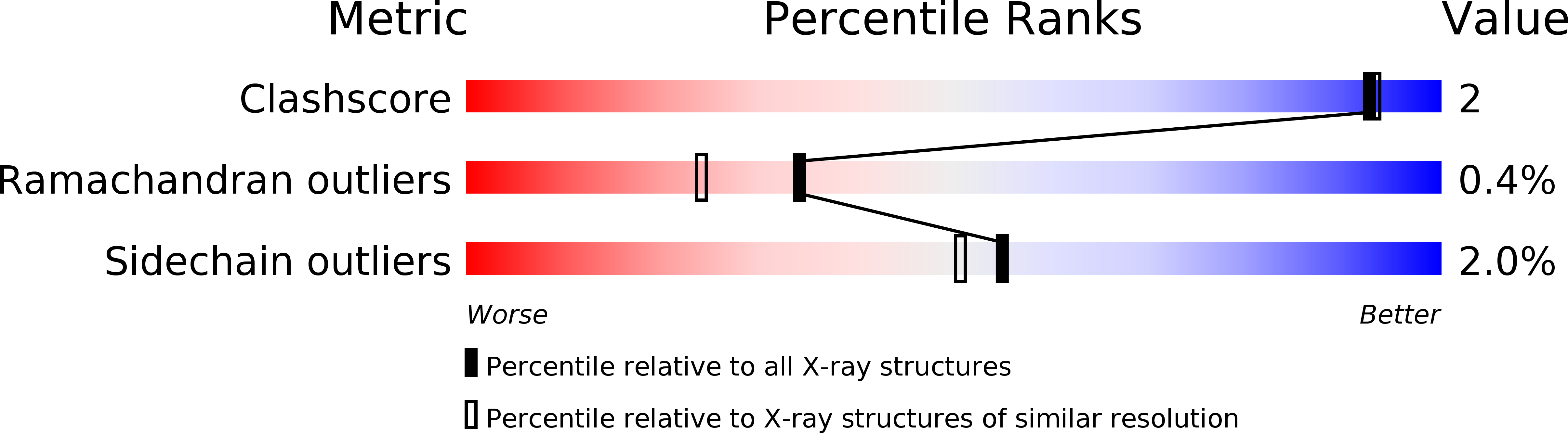
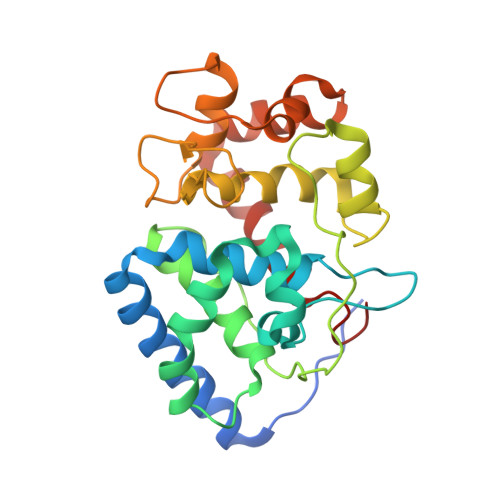
Visualizing the protons in a metalloenzyme electron proton transfer pathway.
Kwon, H., Basran, J., Devos, J.M., Suardiaz, R., van der Kamp, M.W., Mulholland, A.J., Schrader, T.E., Ostermann, A., Blakeley, M.P., Moody, P.C.E., Raven, E.L.(2020) Proc Natl Acad Sci U S A 117: 6484-6490
- PubMed: 32152099
- DOI: https://doi.org/10.1073/pnas.1918936117
- Primary Citation of Related Structures:
6TAE, 6XV4 - PubMed Abstract:
In redox metalloenzymes, the process of electron transfer often involves the concerted movement of a proton. These processes are referred to as proton-coupled electron transfer, and they underpin a wide variety of biological processes, including respiration, energy conversion, photosynthesis, and metalloenzyme catalysis. The mechanisms of proton delivery are incompletely understood, in part due to an absence of information on exact proton locations and hydrogen bonding structures in a bona fide metalloenzyme proton pathway. Here, we present a 2.1-Å neutron crystal structure of the complex formed between a redox metalloenzyme (ascorbate peroxidase) and its reducing substrate (ascorbate). In the neutron structure of the complex, the protonation states of the electron/proton donor (ascorbate) and all of the residues involved in the electron/proton transfer pathway are directly observed. This information sheds light on possible proton movements during heme-catalyzed oxygen activation, as well as on ascorbate oxidation.
Organizational Affiliation:
School of Chemistry, University of Bristol, Bristol BS8 1TS, United Kingdom.