Identification of CNS-Penetrant Aryl Sulfonamides as Isoform-Selective NaV1.6 Inhibitors with Efficacy in Mouse Models of Epilepsy.
Focken, T., Burford, K., Grimwood, M.E., Zenova, A., Andrez, J.C., Gong, W., Wilson, M., Taron, M., Decker, S., Lofstrand, V., Chowdhury, S., Shuart, N., Lin, S., Goodchild, S.J., Young, C., Soriano, M., Tari, P.K., Waldbrook, M., Nelkenbrecher, K., Kwan, R., Lindgren, A., de Boer, G., Lee, S., Sojo, L., DeVita, R.J., Cohen, C.J., Wesolowski, S.S., Johnson Jr., J.P., Dehnhardt, C.M., Empfield, J.R.(2019) J Med Chem 62: 9618-9641
- PubMed: 31525968
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01032
- Primary Citation of Related Structures:
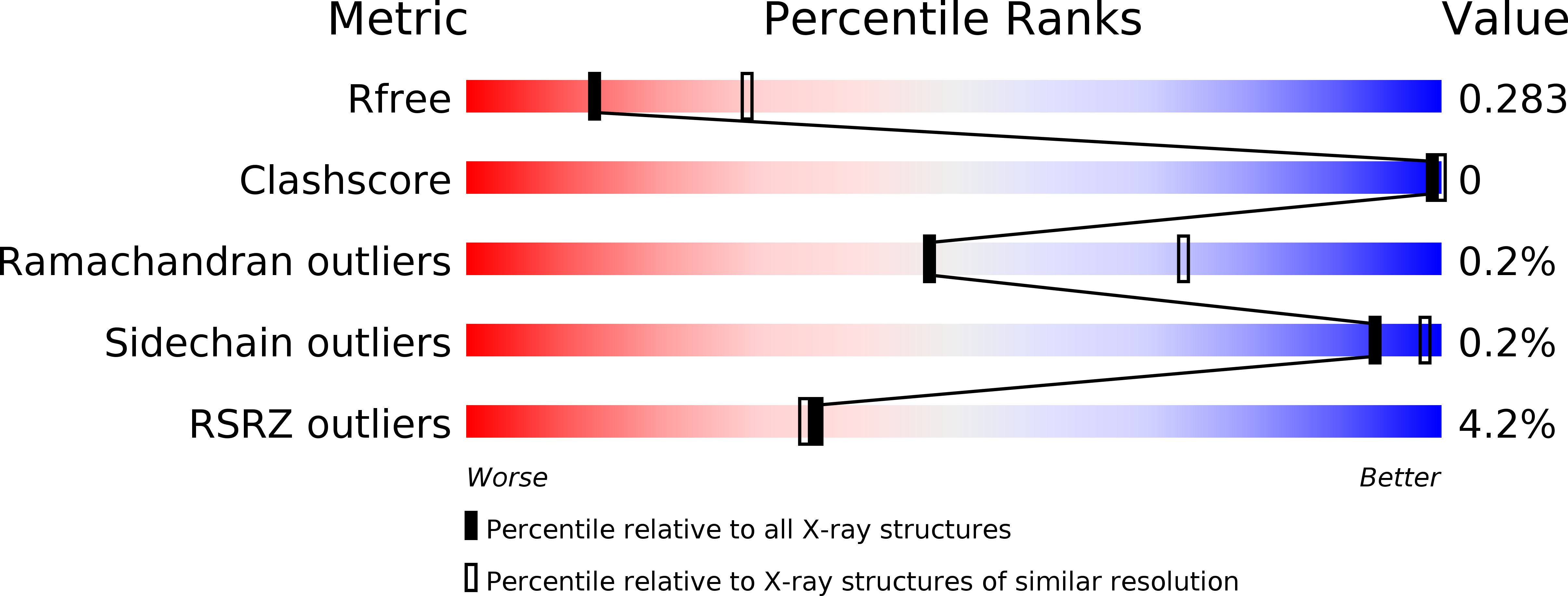
6S41 - PubMed Abstract:
Nonselective antagonists of voltage-gated sodium (Na V ) channels have been long used for the treatment of epilepsies. The efficacy of these drugs is thought to be due to the block of sodium channels on excitatory neurons, primarily Na V 1.6 and Na V 1.2. However, these currently marketed drugs require high drug exposure and suffer from narrow therapeutic indices. Selective inhibition of Na V 1.6, while sparing Na V 1.1, is anticipated to provide a more effective and better tolerated treatment for epilepsies. In addition, block of Na V 1.2 may complement the anticonvulsant activity of Na V 1.6 inhibition. We discovered a novel series of aryl sulfonamides as CNS-penetrant, isoform-selective Na V 1.6 inhibitors, which also displayed potent block of Na V 1.2. Optimization focused on increasing selectivity over Na V 1.1, improving metabolic stability, reducing active efflux, and addressing a pregnane X-receptor liability. We obtained compounds 30-32 , which produced potent anticonvulsant activity in mouse seizure models, including a direct current maximal electroshock seizure assay.
Organizational Affiliation:
Xenon Pharmaceuticals Inc. , 200-3650 Gilmore Way , Burnaby , British Columbia V5G 4W8 , Canada.