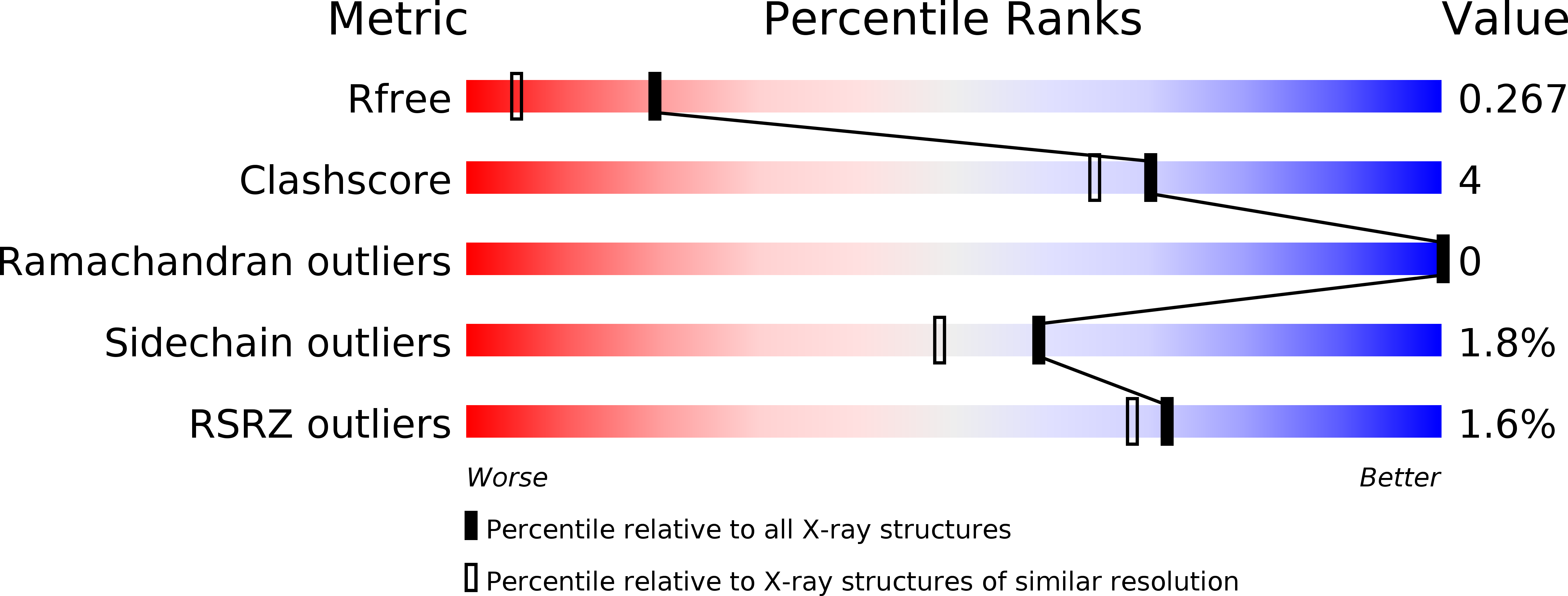
Mapping the Allosteric Communication Network of Aminodeoxychorismate Synthase.
Semmelmann, F., Straub, K., Nazet, J., Rajendran, C., Merkl, R., Sterner, R.(2019) J Mol Biol 431: 2718-2728
- PubMed: 31121180
- DOI: https://doi.org/10.1016/j.jmb.2019.05.021
- Primary Citation of Related Structures:
6QUR - PubMed Abstract:
Allosteric communication between different subunits in metabolic enzyme complexes is of utmost physiological importance but only understood for few systems. We analyzed the structural basis of allostery in aminodeoxychorismate synthase (ADCS), which is a member of the family of glutamine amidotransferases and catalyzes the committed step of the folate biosynthetic pathway. ADCS consists of the synthase subunit PabB and the glutaminase subunit PabA, which is allosterically stimulated by the presence of the PabB substrate chorismate. We first solved the crystal structure of a PabA subunit at 1.9-Å resolution. Based on this structure and the known structure of PabB, we computed an atomic model for the ADCS complex. We then used alanine scanning to test the functional role of 59 conserved residues located between the active sites of PabB and PabA. Steady-state kinetic characterization revealed four branches of a conserved network of mainly charged residues that propagate the signal from chorismate at the PabB active site to the PabA active site. The branches eventually lead to activity-inducing transformations at (i) the oxyanion hole motif, (ii) the catalytic Cys-His-Glu triad, and (iii) glutamine binding residues at the PabA active site. We compare our findings with previously postulated activation mechanisms of different glutamine amidotransferases and propose a unifying regulation mechanism for this ubiquitous family of enzymes.
Organizational Affiliation:
Institute of Biophysics and Physical Biochemistry, University of Regensburg, D-93040 Regensburg, Germany.