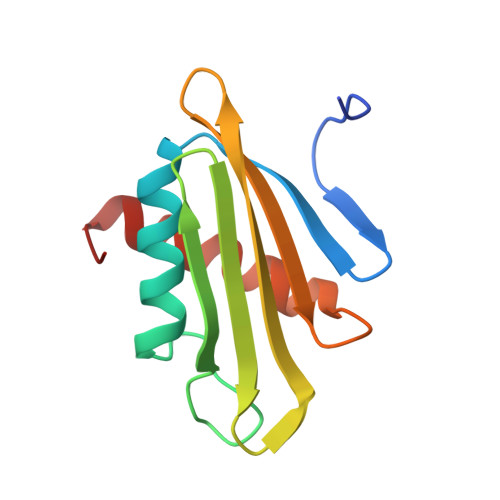
XFEL and NMR Structures of Francisella Lipoprotein Reveal Conformational Space of Drug Target against Tularemia.
Zook, J., Shekhar, M., Hansen, D., Conrad, C., Grant, T., Gupta, C., White, T., Barty, A., Basu, S., Zhao, Y., Zatsepin, N., Ishchenko, A., Batyuk, A., Gati, C., Li, C., Galli, L., Coe, J., Hunter, M., Liang, M., Weierstall, U., Nelson, G., James, D., Stauch, B., Craciunescu, F., Thifault, D., Liu, W., Cherezov, V., Singharoy, A., Fromme, P.(2020) Structure 28: 540
- PubMed: 32142641
- DOI: https://doi.org/10.1016/j.str.2020.02.005
- Primary Citation of Related Structures:
6PNY - PubMed Abstract:
Francisella tularensis is the causative agent for the potentially fatal disease tularemia. The lipoprotein Flpp3 has been identified as a virulence determinant of tularemia with no sequence homology outside the Francisella genus. We report a room temperature structure of Flpp3 determined by serial femtosecond crystallography that exists in a significantly different conformation than previously described by the NMR-determined structure. Furthermore, we investigated the conformational space and energy barriers between these two structures by molecular dynamics umbrella sampling and identified three low-energy intermediate states, transitions between which readily occur at room temperature. We have also begun to investigate organic compounds in silico that may act as inhibitors to Flpp3. This work paves the road to developing targeted therapeutics against tularemia and aides in our understanding of the disease mechanisms of tularemia.
Organizational Affiliation:
Center for Applied Structural Discovery, the Biodesign Institute, Arizona State University, Tempe, AZ 85287, USA.