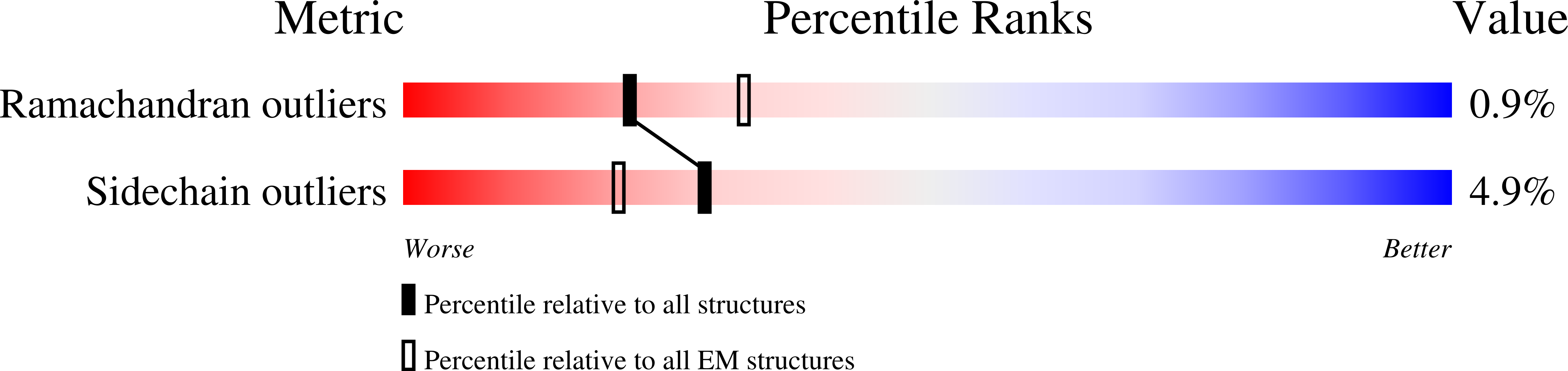Protease-Activatable Adeno-Associated Virus Vector for Gene Delivery to Damaged Heart Tissue.
Guenther, C.M., Brun, M.J., Bennett, A.D., Ho, M.L., Chen, W., Zhu, B., Lam, M., Yamagami, M., Kwon, S., Bhattacharya, N., Sousa, D., Evans, A.C., Voss, J., Sevick-Muraca, E.M., Agbandje-McKenna, M., Suh, J.(2019) Mol Ther 27: 611-622
- PubMed: 30772143
- DOI: https://doi.org/10.1016/j.ymthe.2019.01.015
- Primary Citation of Related Structures:
6NXE - PubMed Abstract:
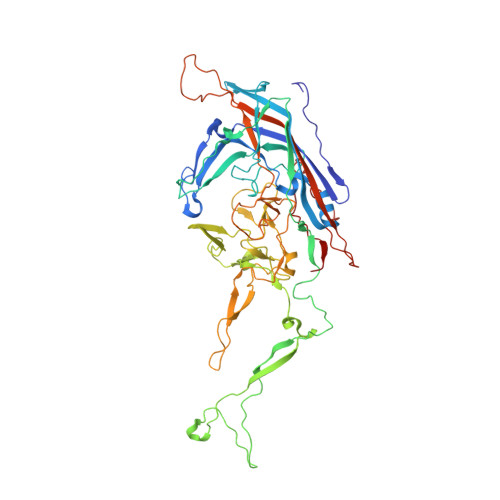
Adeno-associated virus (AAV) has emerged as a promising gene delivery vector because of its non-pathogenicity, simple structure and genome, and low immunogenicity compared to other viruses. However, its adoption as a safe and effective delivery vector for certain diseases relies on altering its tropism to deliver transgenes to desired cell populations. To this end, we have developed a protease-activatable AAV vector, named provector, that responds to elevated extracellular protease activity commonly found in diseased tissue microenvironments. The AAV9-based provector is initially inactive, but then it can be switched on by matrix metalloproteinases (MMP)-2 and -9. Cryo-electron microscopy and image reconstruction reveal that the provector capsid is structurally similar to that of AAV9, with a flexible peptide insertion at the top of the 3-fold protrusions. In an in vivo model of myocardial infarction (MI), the provector is able to deliver transgenes site specifically to high-MMP-activity regions of the damaged heart, with concomitant decreased delivery to many off-target organs, including the liver. The AAV provector may be useful in the future for enhanced delivery of transgenes to sites of cardiac damage.
Organizational Affiliation:
Department of Bioengineering, Rice University, 6100 Main St., Houston, TX 77005, USA.