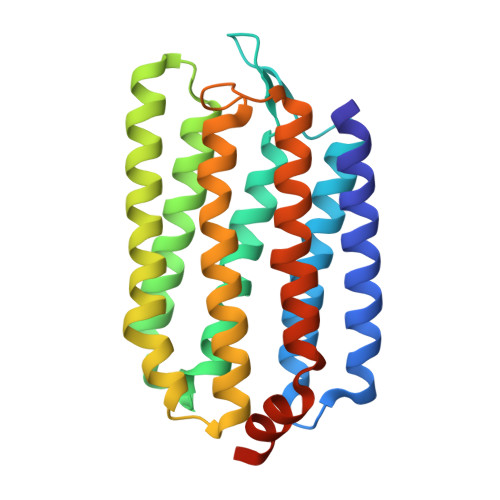
How Does a Microbial Rhodopsin RxR Realize Its Exceptionally High Thermostability with the Proton-Pumping Function Being Retained?
Hayashi, T., Yasuda, S., Suzuki, K., Akiyama, T., Kanehara, K., Kojima, K., Tanabe, M., Kato, R., Senda, T., Sudo, Y., Murata, T., Kinoshita, M.(2020) J Phys Chem B 124: 990-1000
- PubMed: 31955569
- DOI: https://doi.org/10.1021/acs.jpcb.9b10700
- Primary Citation of Related Structures:
6KFQ - PubMed Abstract:
We often encounter a case where two proteins, whose amino-acid sequences are similar, are quite different with regard to the thermostability. As a striking example, we consider the two seven-transmembrane proteins: recently discovered Rubrobacter xylanophilus rhodopsin (RxR) and long-known bacteriorhodopsin from Halobacterium salinarum (HsBR). They commonly function as a light-driven proton pump across the membrane. Though their sequence similarity and identity are ∼71 and ∼45%, respectively, RxR is much more thermostable than HsBR. In this study, we solve the three-dimensional structure of RxR using X-ray crystallography and find that the backbone structures of RxR and HsBR are surprisingly similar to each other: The root-mean-square deviation for the two structures calculated using the backbone C α atoms of the seven helices is only 0.86 Å, which makes the large stability difference more puzzling. We calculate the thermostability measure and its energetic and entropic components for RxR and HsBR using our recently developed statistical-mechanical theory. The same type of calculation is independently performed for the portions playing essential roles in the proton-pumping function, helices 3 and 7, and their structural properties are related to the probable roles of water molecules in the proton-transporting mechanism. We succeed in elucidating how RxR realizes its exceptionally high stability with the original function being retained. This study provides an important first step toward the establishment of a method correlating microscopic, geometric characteristics of a protein with its thermodynamic properties and enhancing the thermostability through amino-acid mutations without vitiating the original function.
Organizational Affiliation:
Institute of Advanced Energy , Kyoto University , Gokasho, Uji, Kyoto 611-0011 , Japan.