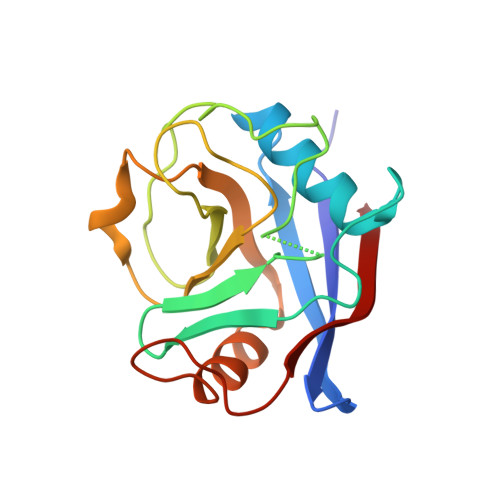
Dynamic design: manipulation of millisecond timescale motions on the energy landscape of cyclophilin A
Juarez-Jimenez, J., Gupta, A.A., Karunanithy, G., May, A., Georgiou, C., Ioannidis, H., De Simone, A., Barlow, P.N., Hulme, A.N., Walkinshaw, M.D., Baldwin, A., Michel, J.(2020) Chem Sci