Monoclinic crystalline form of human insulin, complexed with meta-cresol
Margiolaki, I., Karavassili, F., Valmas, A., Dimarogona, M., Giannopoulou, A.E., Fili, S., Schluckebier, G., Norrman, M., Beckers, D., Fitch, A.N.To be published.
Experimental Data Snapshot
Find similar proteins by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Insulin | 21 | Homo sapiens | Mutation(s): 0 Gene Names: INS | 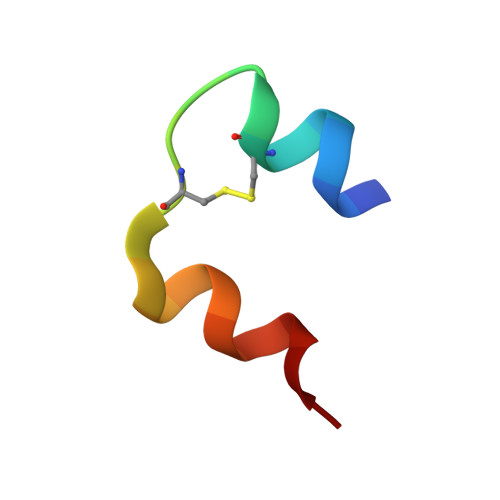 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01308 (Homo sapiens) Explore P01308 Go to UniProtKB: P01308 | |||||
PHAROS: P01308 GTEx: ENSG00000254647 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01308 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Insulin | 30 | Homo sapiens | Mutation(s): 0 Gene Names: INS | 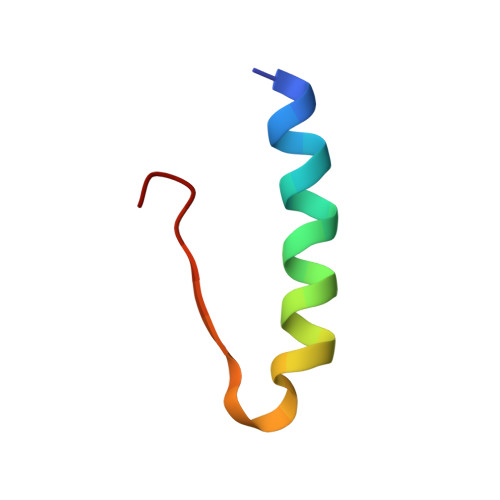 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01308 (Homo sapiens) Explore P01308 Go to UniProtKB: P01308 | |||||
PHAROS: P01308 GTEx: ENSG00000254647 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01308 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CRS (Subject of Investigation/LOI) Query on CRS | AB [auth U] BB [auth W] CA [auth C] GA [auth E] JA [auth G] | M-CRESOL C7 H8 O RLSSMJSEOOYNOY-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | AA [auth B], DA [auth D], PA [auth N], SA [auth P] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | EA [auth D] HA [auth E] IA [auth F] KA [auth H] LA [auth H] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| IS8 Query on IS8 | BA [auth B], FA [auth D], QA [auth N], UA [auth P] | isothiocyanate C H N S GRHBQAYDJPGGLF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.662 | α = 90 |
| b = 70.361 | β = 105.21 |
| c = 84.748 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Union | Greece | BioStruct-X,No.283570 |
| "General Secretariat for Research and Technology (GSRT) - Hellenic Foundation for Research and Innovation | Greece | 2467 |