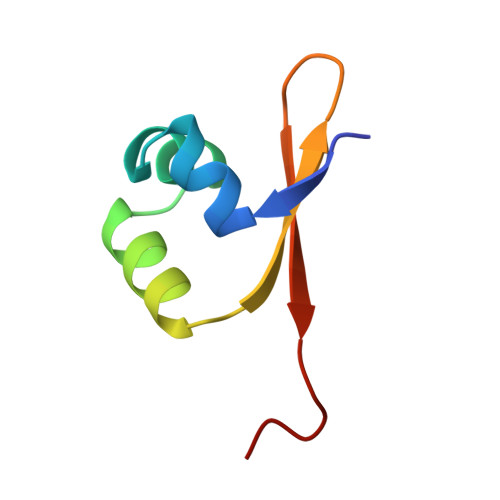
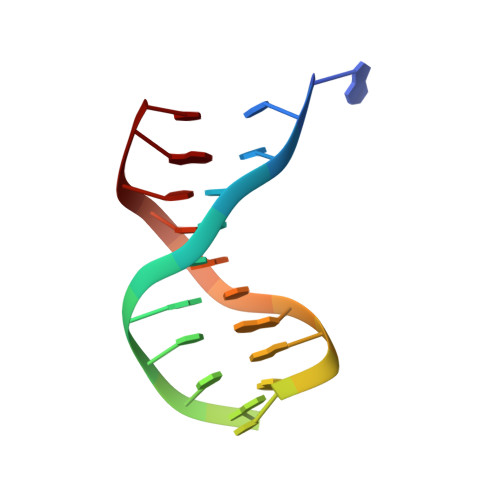
Crystal structure of lambda-Cro bound to a consensus operator at 3.0 A resolution.
Albright, R.A., Matthews, B.W.(1998) J Mol Biol 280: 137-151
- PubMed: 9653037
- DOI: https://doi.org/10.1006/jmbi.1998.1848
- Primary Citation of Related Structures:
6CRO - PubMed Abstract:
The structure of the Cro protein from bacteriophage lambda in complex with a 19 base-pair DNA duplex that includes the 17 base-pair consensus operator has been determined at 3.0 A resolution. The structure confirms the large changes in the protein and DNA seen previously in a crystallographically distinct low-resolution structure of the complex and, for the first time, reveals the detailed interactions between the side-chains of the protein and the base-pairs of the operator. Relative to the crystal structure of the free protein, the subunits of Cro rotate 53 degrees with respect to each other on binding DNA. At the same time the DNA is bent by 40 degrees through the 19 base-pairs. The intersubunit connection includes a region within the protein core that is structurally reminiscent of the "ball and socket" motif seen in the immunoglobulins and T-cell receptors. The crystal structure of the Cro complex is consistent with virtually all available biochemical and related data. Some of the interactions between Cro and DNA proposed on the basis of model-building are now seen to be correct, but many are different. Tests of the original model by mutagenesis and biochemical analysis corrected some but not all of the errors. Within the limitations of the crystallographic resolution it appears that operator recognition is achieved almost entirely by direct hydrogen-bonding and van der Waals contacts between the protein and the exposed bases within the major groove of the DNA. The discrimination of Cro between the operators OR3 and OR1, which differ in sequence at just three positions, is inferred to result from a combination of small differences, both favorable and unfavorable. A van der Waals contact at one of the positions is of primary importance, while the other two provide smaller, indirect effects. Direct hydrogen bonding is not utilized in this distinction.
Organizational Affiliation:
Institute of Molecular Biology Howard Hughes Medical Institute and Department of Physics, University of Oregon, Eugene, OR, 97403-1229, USA.