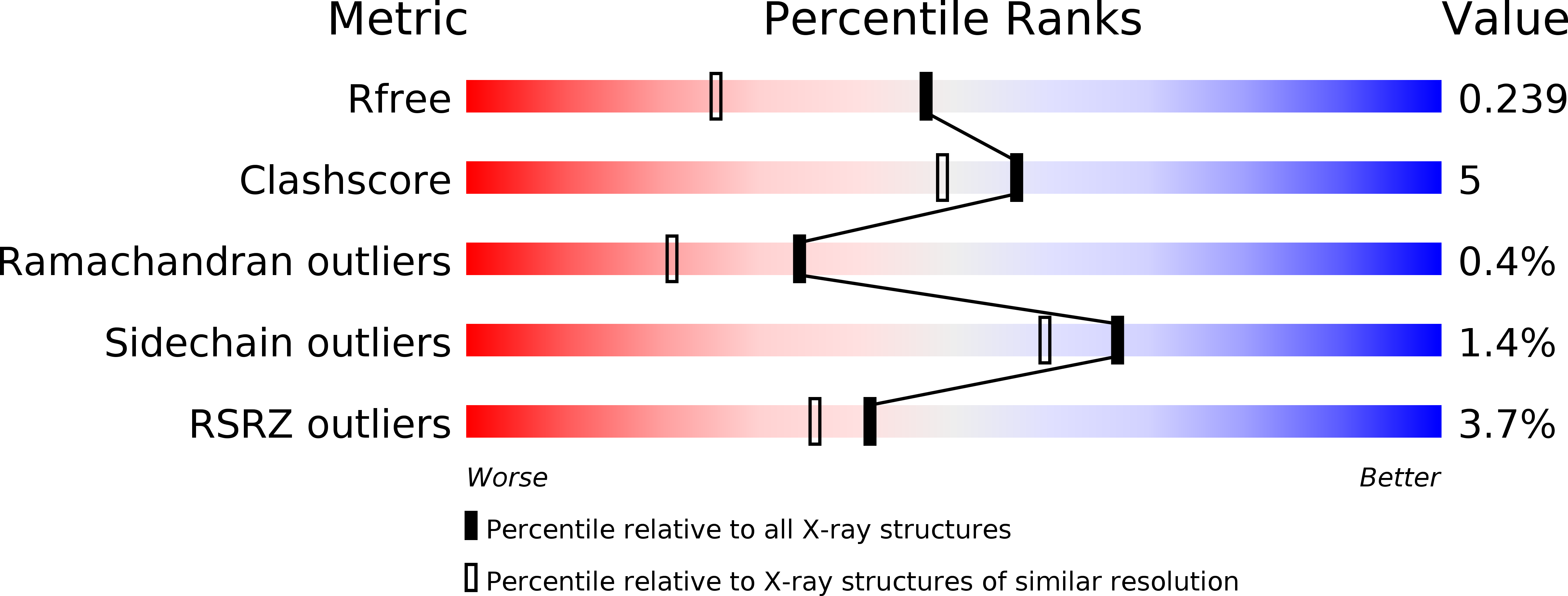
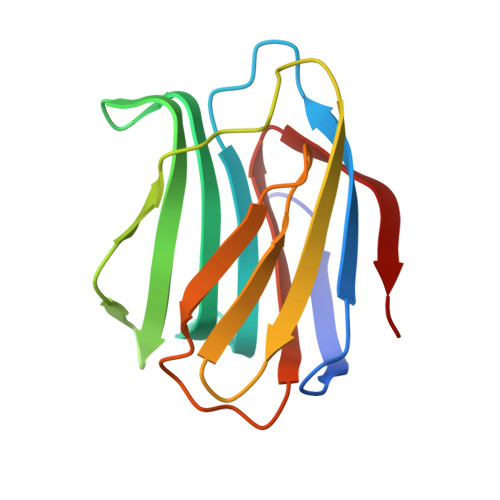
Lactulose as a novel template for anticancer drug development targeting galectins.
Kishor, C., Ross, R.L., Blanchard, H.(2018) Chem Biol Drug Des 92: 1801-1808
- PubMed: 29888844
- DOI: https://doi.org/10.1111/cbdd.13348
- Primary Citation of Related Structures:
6B8K, 6B94 - PubMed Abstract:
Galectins are carbohydrate binding proteins (lectins), which characteristically bind β-galactosides. Galectins play a role in tumour progression through involvement in proliferation, metastasis, angiogenesis, immune evasion and drug resistance. There is need for inhibitors (antagonists) that are specific for distinct galectins and that can interfere with galectin-carbohydrate interactions during cancer progression. Here, we propose that lactulose, a non-digestible galactose-fructose disaccharide, presents a novel inhibitor scaffold for design of inhibitors against galectins. Thermodynamic evaluation displays binding affinity of lactulose against the galectin-1 and galectin-3 carbohydrate recognition domain (CRD). Crystal structures of galectin-1 and galectin-3 in complex with lactulose reveal for the first time the molecular basis of the galectin-lactulose interactions. Molecular modelling was implemented to propose novel lactulose derivatives as potent anti-cancer agents.
Organizational Affiliation:
Institute for Glycomics, Griffith University, Gold Coast, QLD, Australia.