Protein engineering for improving the thermostability of tryptophan oxidase and insights from structural analysis.
Yamaguchi, H., Tatsumi, M., Takahashi, K., Tagami, U., Sugiki, M., Kashiwagi, T., Kameya, M., Okazaki, S., Mizukoshi, T., Asano, Y.(2018) J Biochem 164: 359-367
- PubMed: 30053101
- DOI: https://doi.org/10.1093/jb/mvy065
- Primary Citation of Related Structures:
5ZBC, 5ZBD - PubMed Abstract:
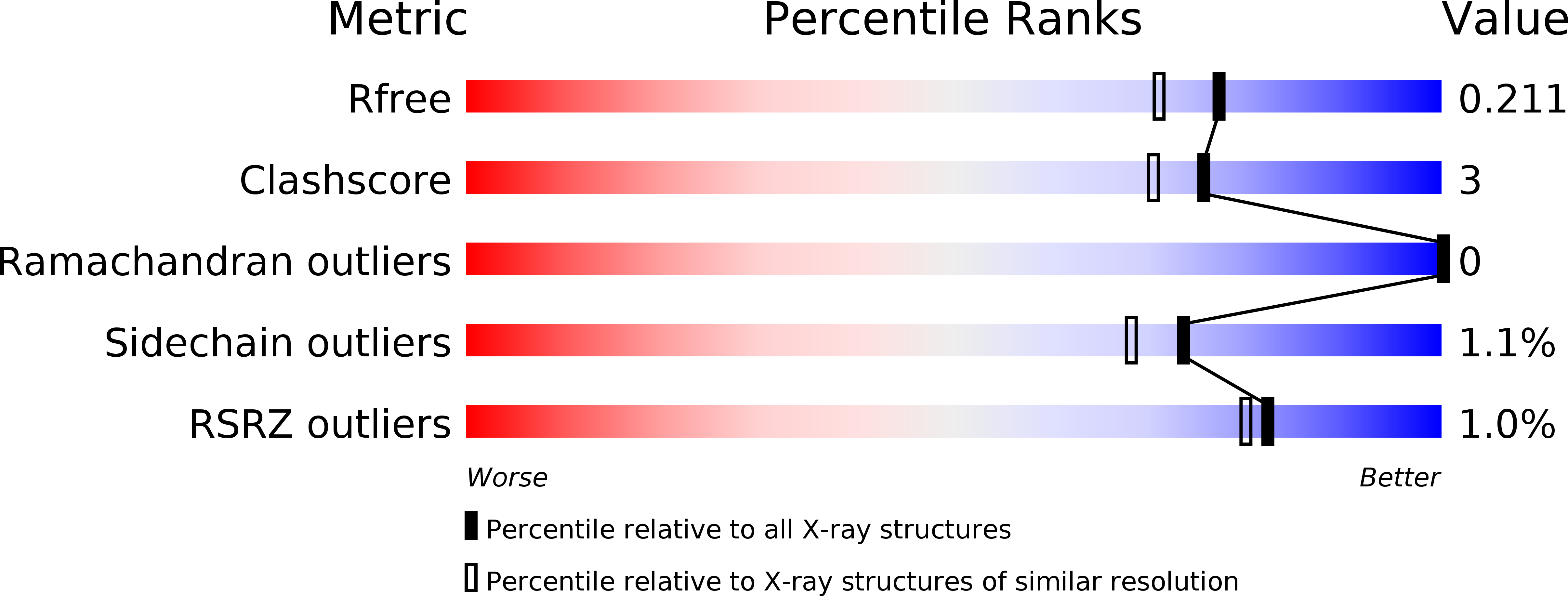
l-Tryptophan oxidase, VioA from Chromobacterium violaceum, which has a high substrate specificity for tryptophan, is useful for quantitative assay of tryptophan. However, stability of wild type VioA is not enough for its application in clinical or industrial use. To improve the thermal stability of the enzyme, we developed a VioA (C395A) mutant, with higher stability than wild type VioA. The VioA (C395A) exhibited similar specificity and kinetic parameter for tryptophan to wild type. Conventionally, the quantity of tryptophan is determined by instrumental methods, such as high-performance liquid chromatography (HPLC) after pre-column-derivatization. Using the mutant enzyme, we succeeded in the tryptophan quantification in human plasma samples, to an accuracy of <2.9% when compared to the instrumental method, and to a precision of CV <3.2%. To analyse the improvement in storage stability and substrate specificity, we further determined the crystal structures of VioA (C395A) complexed with FAD, and with FAD and tryptophan at 1.8 Å resolution.
Organizational Affiliation:
Fundamental Technology Labs., Institute for Innovation, Ajinomoto Co., Inc., Kawasaki, Japan.