Structural plasticity of the TDRD3 Tudor domain probed by a fragment screening hit.
Liu, J., Zhang, S., Liu, M., Liu, Y., Nshogoza, G., Gao, J., Ma, R., Yang, Y., Wu, J., Zhang, J., Li, F., Ruan, K.(2018) FEBS J 285: 2091-2103
- PubMed: 29645362
- DOI: https://doi.org/10.1111/febs.14469
- Primary Citation of Related Structures:
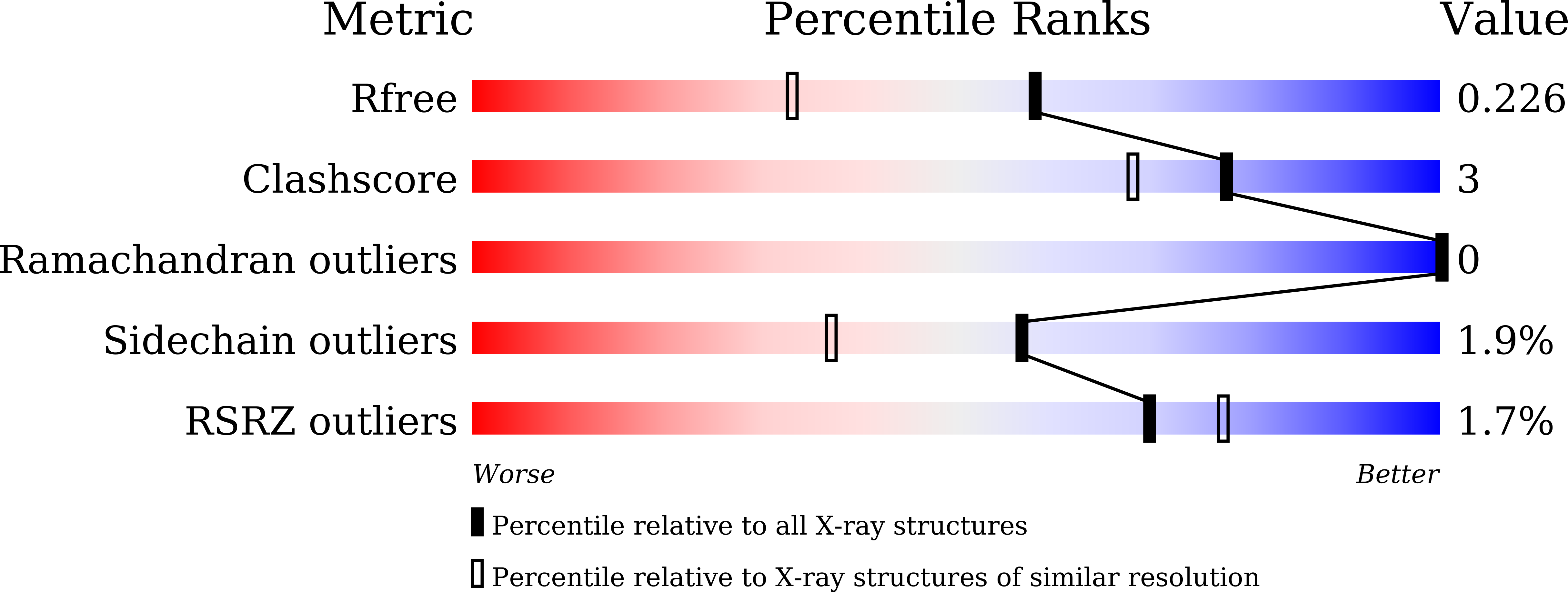
5YJ8 - PubMed Abstract:
As a reader of di-methylated arginine on various proteins, such as histone, RNA polymerase II, PIWI and Fragile X mental retardation protein, the Tudor domain of Tudor domain-containing protein 3 (TDRD3) mediates transcriptional activation in nucleus and formation of stress granules in the cytoplasm. Despite the TDRD3 implication in cancer cell proliferation and invasion, warheads to block the di-methylated arginine recognition pocket of the TDRD3 Tudor domain have not yet been uncovered. Here we identified 14 small molecule hits against the TDRD3 Tudor domain through NMR fragment-based screening. These hits were further cross-validated by using competitive fluorescence polarization and isothermal titration calorimetry experiments. The crystal structure of the TDRD3 Tudor domain in complex with hit 1 reveals a distinct binding mode from the nature substrate. Hit 1 protrudes into the aromatic cage of the TDRD3 Tudor domain, where the aromatic residues are tilted to accommodate a sandwich-like π-π interaction. The side chain of the conserved residue N596 swings away 3.1 Å to form a direct hydrogen bond with hit 1. Moreover, this compound shows a decreased affinity against the single Tudor domain of survival motor neuron protein, but no detectable binding to neither the tandem Tudor domain of TP53-binding protein 1 nor the extended Tudor domain of staphylococcal nuclease domain-containing protein 1. Our work depicts the structural plasticity of the TDRD3 Tudor domain and paves the way for the subsequent structure-guided discovery of selective inhibitors targeting Tudor domains.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at the Microscale, School of Life Sciences, University of Science and Technology of China, Hefei, China.