Staphylococcus aureus single-stranded DNA-binding protein SsbA can bind but cannot stimulate PriA helicase.
Huang, Y.H., Guan, H.H., Chen, C.J., Huang, C.Y.(2017) PLoS One 12: e0182060-e0182060
- PubMed: 28750050
- DOI: https://doi.org/10.1371/journal.pone.0182060
- Primary Citation of Related Structures:
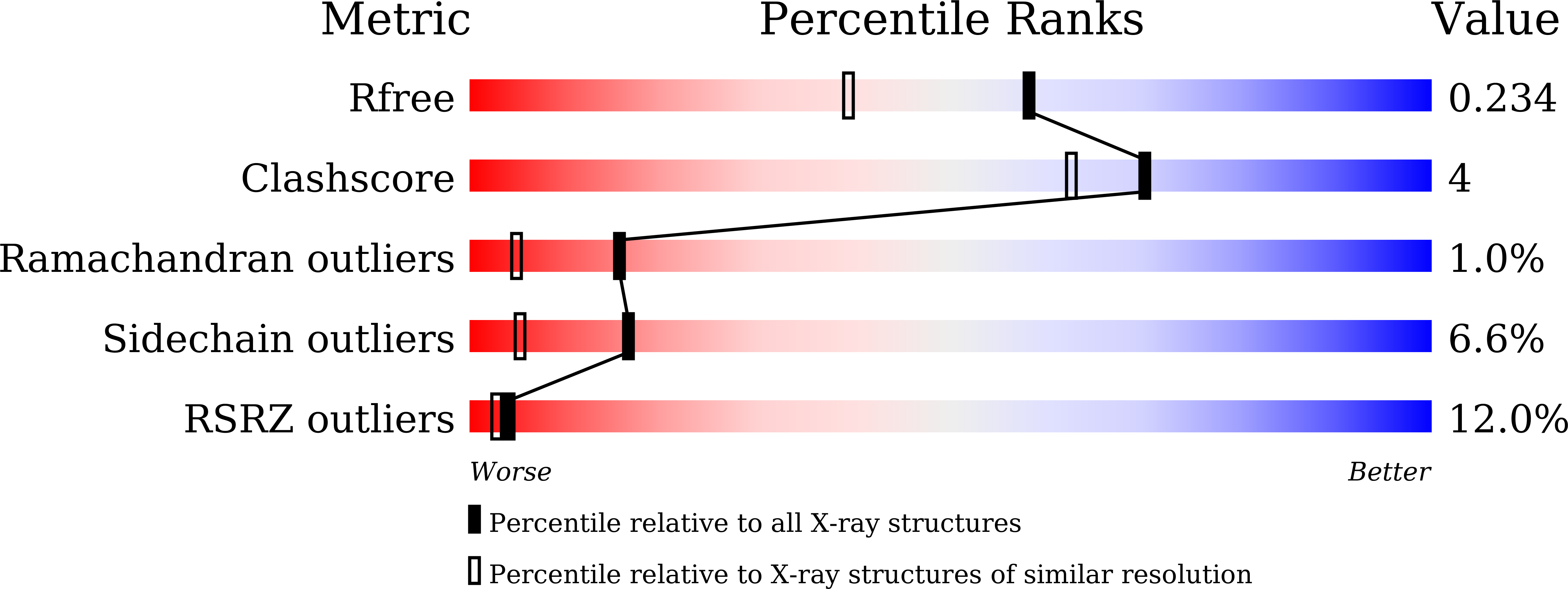
5XGT - PubMed Abstract:
Single-stranded DNA-binding protein (SSB) and PriA helicase play important roles in bacterial DNA replication restart process. The mechanism by which PriA helicase is bound and stimulated by SSB in Escherichia coli (Ec) has been established, but information on this process in Gram-positive bacteria are limited. We characterized the properties of SSB from Staphylococcus aureus (SaSsbA, a counterpart of EcSSB) and analyzed its interaction with SaPriA. The gel filtration chromatography analysis of purified SaSsbA showed a stable tetramer in solution. The crystal structure of SaSsbA determined at 1.82 Å resolution (PDB entry 5XGT) reveals that the classic oligonucleotide/oligosaccharide-binding folds are formed in the N-terminal DNA-binding domain, but the entire C-terminal domain is disordered. Unlike EcSSB, which can stimulate EcPriA via a physical interaction between EcPriA and the C-terminus of EcSSB (SSB-Ct), SaSsbA does not affect the activity of SaPriA. We also found that SaPriA can be bound by SaSsbA, but not by SaSsbA-Ct. Although no effect was found with SaSsbA, SaPriA can be significantly stimulated by the Gram-negative Klebsiella pneumoniae SSB (KpSSB). In addition, we found that the conserved SSB-Ct binding site of KpPriA (Trp82, Tyr86, Lys370, Arg697, and Gln701) is not present in SaPriA. Arg697 in KpPriA is known to play a critical role in altering the SSB35/SSB65 distribution, but this corresponding residue in SaPriA is Glu767 instead, which has an opposite charge to Arg. SaPriA E767R mutant was constructed and analyzed; however, it still cannot be stimulated by SaSsbA. Finally, we found that the conserved MDFDDDIPF motif in the Gram-negative bacterial SSB is DISDDDLPF in SaSsbA, i.e., F172 in EcSSB and F168 in KpSSB is S161 in SaSsbA, not F. When acting with SaSsbA S161F mutant, the activity of SaPriA was dramatically enhanced elevenfold. Overall, the conserved binding sites, both in EcPriA and EcSSB, are not present in SaPriA and SaSsbA, thereby no stimulation occurs. Our observations through structure-sequence comparison and mutational analyses indicate that the case of EcPriA-EcSSB is not applicable to SaPriA-SaSsbA because of inherent differences among the species.
Organizational Affiliation:
School of Biomedical Sciences, Chung Shan Medical University, Taichung City, Taiwan.