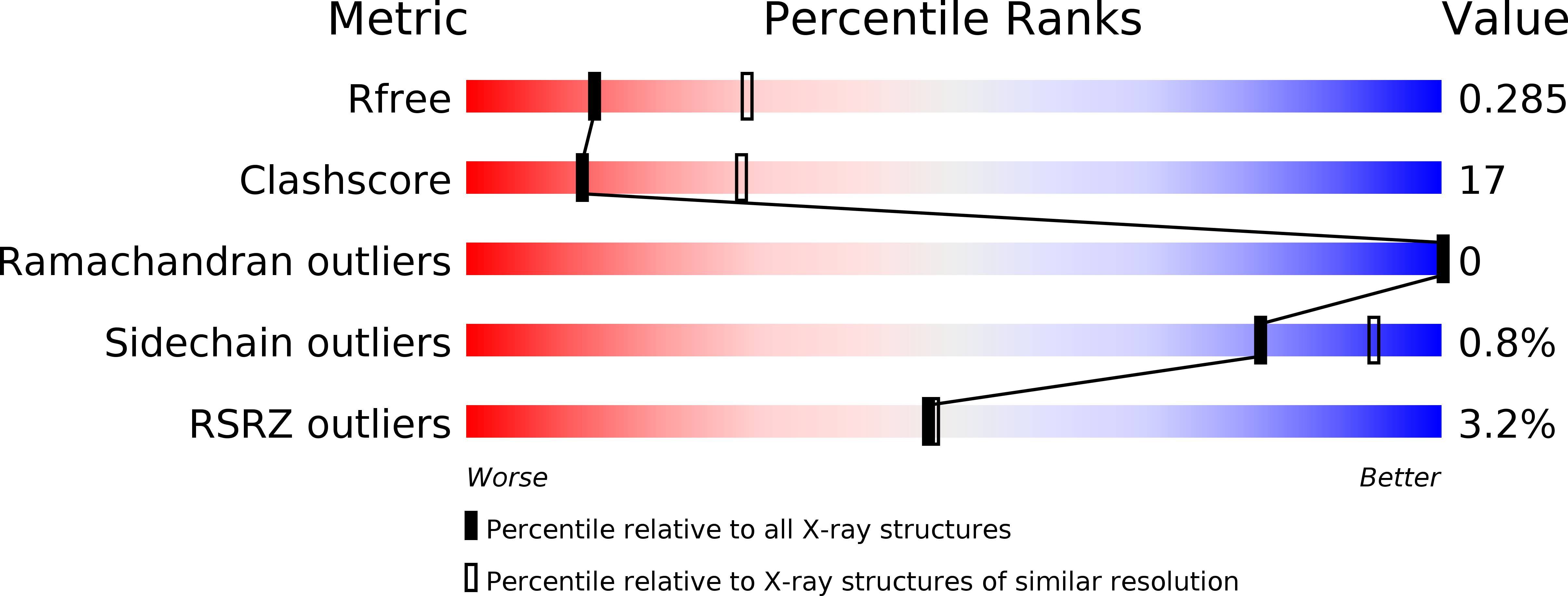
Molecular Basis for Cohesin Acetylation by Establishment of Sister Chromatid Cohesion N-Acetyltransferase ESCO1.
Rivera-Colon, Y., Maguire, A., Liszczak, G.P., Olia, A.S., Marmorstein, R.(2016) J Biol Chem 291: 26468-26477
- PubMed: 27803161
- DOI: https://doi.org/10.1074/jbc.M116.752220
- Primary Citation of Related Structures:
5T53 - PubMed Abstract:
Protein acetylation is a prevalent posttranslational modification that is regulated by diverse acetyltransferase enzymes. Although histone acetyltransferases (HATs) have been well characterized both structurally and mechanistically, far less is known about non-histone acetyltransferase enzymes. The human ESCO1 and ESCO2 paralogs acetylate the cohesin complex subunit SMC3 to regulate the separation of sister chromatids during mitosis and meiosis. Missense mutations within the acetyltransferase domain of these proteins correlate with diseases, including endometrial cancers and Roberts syndrome. Despite their biological importance, the mechanisms underlying acetylation by the ESCO proteins are not understood. Here, we report the X-ray crystal structure of the highly conserved zinc finger-acetyltransferase moiety of ESCO1 with accompanying structure-based mutagenesis and biochemical characterization. We find that the ESCO1 acetyltransferase core is structurally homologous to the Gcn5 HAT, but contains unique additional features including a zinc finger and an ∼40-residue loop region that appear to play roles in protein stability and SMC3 substrate binding. We identify key residues that play roles in substrate binding and catalysis, and rationalize the functional consequences of disease-associated mutations. Together, these studies reveal the molecular basis for SMC3 acetylation by ESCO1 and have broader implications for understanding the structure/function of non-histone acetyltransferases.
Organizational Affiliation:
From the Department of Biochemistry and Biophysics, Abramson Family Cancer Research Institute, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania 19104 and.