Crystal structure of undecaprenyl-pyrophosphate phosphatase and its role in peptidoglycan biosynthesis.
El Ghachi, M., Howe, N., Huang, C.Y., Olieric, V., Warshamanage, R., Touze, T., Weichert, D., Stansfeld, P.J., Wang, M., Kerff, F., Caffrey, M.(2018) Nat Commun 9: 1078-1078
- PubMed: 29540682
- DOI: https://doi.org/10.1038/s41467-018-03477-5
- Primary Citation of Related Structures:
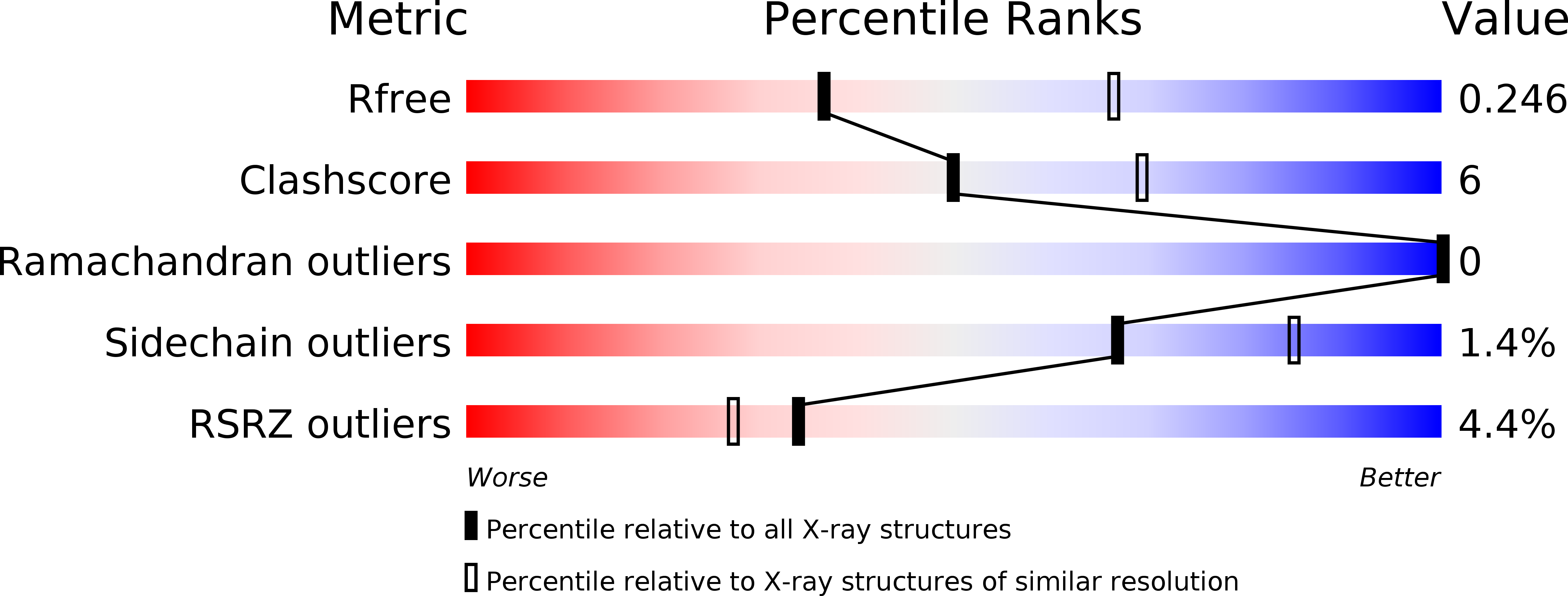
5OON - PubMed Abstract:
As a protective envelope surrounding the bacterial cell, the peptidoglycan sacculus is a site of vulnerability and an antibiotic target. Peptidoglycan components, assembled in the cytoplasm, are shuttled across the membrane in a cycle that uses undecaprenyl-phosphate. A product of peptidoglycan synthesis, undecaprenyl-pyrophosphate, is converted to undecaprenyl-phosphate for reuse in the cycle by the membrane integral pyrophosphatase, BacA. To understand how BacA functions, we determine its crystal structure at 2.6 Å resolution. The enzyme is open to the periplasm and to the periplasmic leaflet via a pocket that extends into the membrane. Conserved residues map to the pocket where pyrophosphorolysis occurs. BacA incorporates an interdigitated inverted topology repeat, a topology type thus far only reported in transporters and channels. This unique topology raises issues regarding the ancestry of BacA, the possibility that BacA has alternate active sites on either side of the membrane and its possible function as a flippase.
Organizational Affiliation:
Centre d'Ingénierie des Protéines, InBioS, Université de Liège, allée du 6 Août 19, Bât B5a, 4000, Liège, Belgium.