A molecular mechanism for the enzymatic methylation of nitrogen atoms within peptide bonds.
Song, H., van der Velden, N.S., Shiran, S.L., Bleiziffer, P., Zach, C., Sieber, R., Imani, A.S., Krausbeck, F., Aebi, M., Freeman, M.F., Riniker, S., Kunzler, M., Naismith, J.H.(2018) Sci Adv 4: eaat2720-eaat2720
- PubMed: 30151425
- DOI: https://doi.org/10.1126/sciadv.aat2720
- Primary Citation of Related Structures:
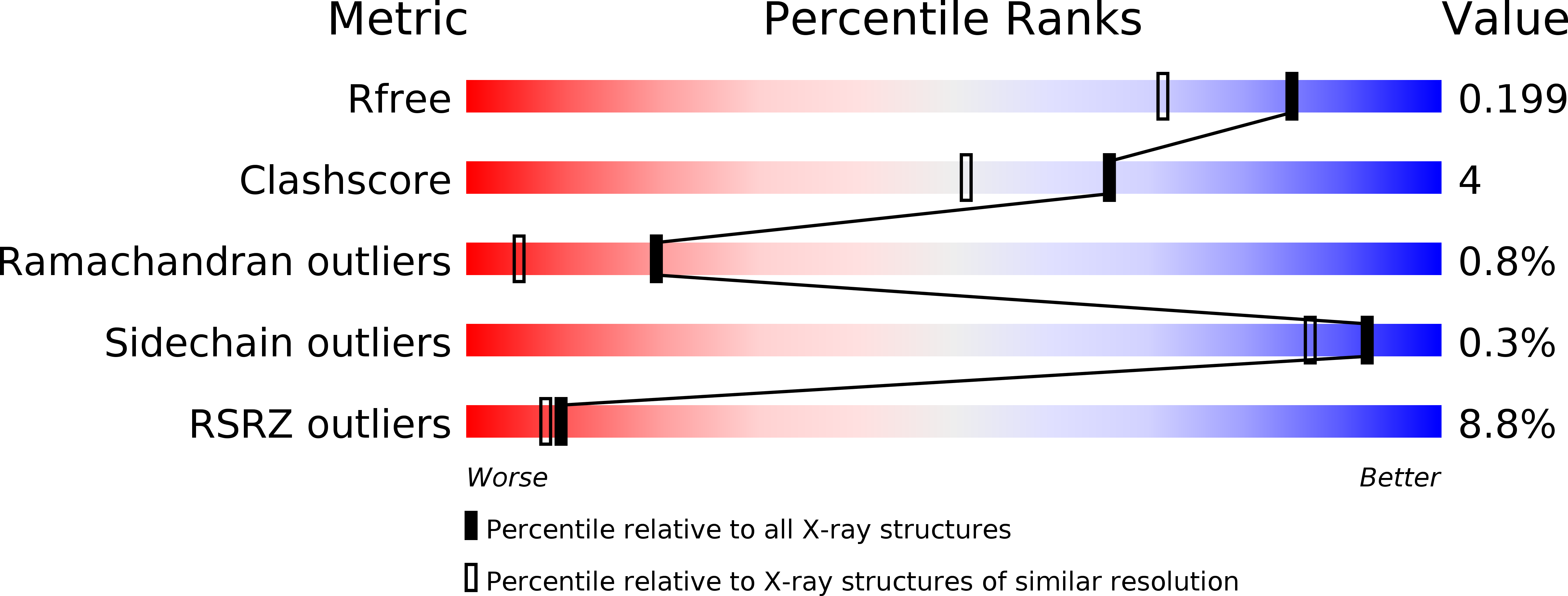
5N0N, 5N0O, 5N0P, 5N0Q, 5N0R, 5N0S, 5N0T, 5N0U, 5N0V, 5N0W, 5N0X, 5N4I, 5OUF, 6GEW - PubMed Abstract:
The peptide bond, the defining feature of proteins, governs peptide chemistry by abolishing nucleophilicity of the nitrogen. This and the planarity of the peptide bond arise from the delocalization of the lone pair of electrons on the nitrogen atom into the adjacent carbonyl. While chemical methylation of an amide bond uses a strong base to generate the imidate, OphA, the precursor protein of the fungal peptide macrocycle omphalotin A, self-hypermethylates amides at pH 7 using S -adenosyl methionine (SAM) as cofactor. The structure of OphA reveals a complex catenane-like arrangement in which the peptide substrate is clamped with its amide nitrogen aligned for nucleophilic attack on the methyl group of SAM. Biochemical data and computational modeling suggest a base-catalyzed reaction with the protein stabilizing the reaction intermediate. Backbone N-methylation of peptides enhances their protease resistance and membrane permeability, a property that holds promise for applications to medicinal chemistry.
Organizational Affiliation:
Biomedical Sciences Research Complex, North Haugh, University of St. Andrews, Fife KY16 9ST, UK.