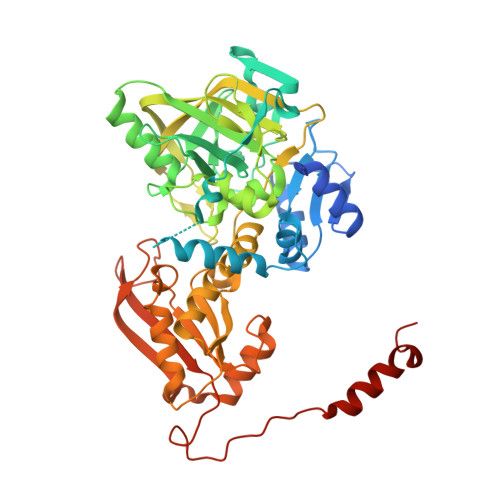
Oxidative Maturation and Structural Characterization of Prenylated FMN Binding by UbiD, a Decarboxylase Involved in Bacterial Ubiquinone Biosynthesis.
Marshall, S.A., Fisher, K., Ni Cheallaigh, A., White, M.D., Payne, K.A., Parker, D.A., Rigby, S.E., Leys, D.(2017) J Biol Chem 292: 4623-4637
- PubMed: 28057757
- DOI: https://doi.org/10.1074/jbc.M116.762732
- Primary Citation of Related Structures:
5M1B, 5M1C, 5M1D, 5M1E - PubMed Abstract:
The activity of the reversible decarboxylase enzyme Fdc1 is dependent on prenylated FMN (prFMN), a recently discovered cofactor. The oxidized prFMN supports a 1,3-dipolar cycloaddition mechanism that underpins reversible decarboxylation. Fdc1 is a distinct member of the UbiD family of enzymes, with the canonical UbiD catalyzing the (de)carboxylation of para -hydroxybenzoic acid-type substrates. Here we show that the Escherichia coli UbiD enzyme, which is implicated in ubiquinone biosynthesis, cannot be isolated in an active holoenzyme form despite the fact active holoFdc1 is readily obtained. Formation of holoUbiD requires reconstitution in vitro of the apoUbiD with reduced prFMN. Furthermore, although the Fdc1 apoenzyme can be readily reconstituted and activated, in vitro oxidation to the mature prFMN cofactor stalls at formation of a radical prFMN species in holoUbiD. Further oxidative maturation in vitro occurs only at alkaline pH, suggesting a proton-coupled electron transfer precedes formation of the fully oxidized prFMN. Crystal structures of holoUbiD reveal a relatively open active site potentially occluded from solvent through domain motion. The presence of a prFMN sulfite-adduct in one of the UbiD crystal structures confirms oxidative maturation does occur at ambient pH on a slow time scale. Activity could not be detected for a range of putative para -hydroxybenzoic acid substrates tested. However, the lack of an obvious hydrophobic binding pocket for the octaprenyl tail of the proposed ubiquinone precursor substrate does suggest UbiD might act on a non-prenylated precursor. Our data reveals an unexpected variation occurs in domain mobility, prFMN binding, and maturation by the UbiD enzyme family.
Organizational Affiliation:
From the Manchester Institute of Biotechnology, University of Manchester, 131 Princess Street Manchester, M1 7DN, United Kingdom and.