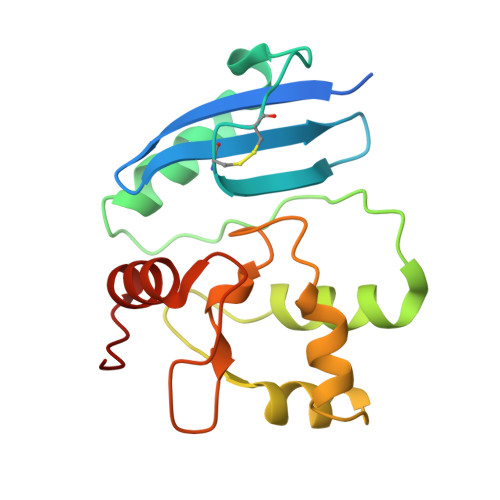
Interdomain interactions rearrangements control the reaction steps of a thermostable DNA alkyltransferase.
Morrone, C., Miggiano, R., Serpe, M., Massarotti, A., Valenti, A., Del Monaco, G., Rossi, M., Rossi, F., Rizzi, M., Perugino, G., Ciaramella, M.(2016) Biochim Biophys Acta 1861: 86-96
- PubMed: 27777086
- DOI: https://doi.org/10.1016/j.bbagen.2016.10.020
- Primary Citation of Related Structures:
5LLQ - PubMed Abstract:
Alkylated DNA-protein alkyltransferases (AGTs) are conserved proteins that repair alkylation damage in DNA by using a single-step mechanism leading to irreversible alkylation of the catalytic cysteine in the active site. Trans-alkylation induces inactivation and destabilization of the protein, both in vitro and in vivo, likely triggering conformational changes. A complete picture of structural rearrangements occurring during the reaction cycle is missing, despite considerable interest raised by the peculiarity of AGT reaction, and the contribution of a functional AGT in limiting the efficacy of chemotherapy with alkylating drugs. As a model for AGTs we have used a thermostable ortholog from the archaeon Sulfolobus solfataricus (SsOGT), performing biochemical, structural, molecular dynamics and in silico analysis of ligand-free, DNA-bound and mutated versions of the protein. Conformational changes occurring during lesion recognition and after the reaction, allowed us to identify a novel interaction network contributing to SsOGT stability, which is perturbed when a bulky adduct between the catalytic cysteine and the alkyl group is formed, a mandatory step toward the permanent protein alkylation. Our data highlighted conformational changes and perturbation of intramolecular interaction occurring during lesion recognition and catalysis, confirming our previous hypothesis that coordination between the N- and C-terminal domains of SsOGT is important for protein activity and stability. A general model of structural rearrangements occurring during the reaction cycle of AGTs is proposed. If confirmed, this model might be a starting point to design strategies to modulate AGT activity in therapeutic settings.
Organizational Affiliation:
Institute of Biosciences and BioResources, National Research Council of Italy, Via P. Castellino 111, 80125 Naples, Italy.