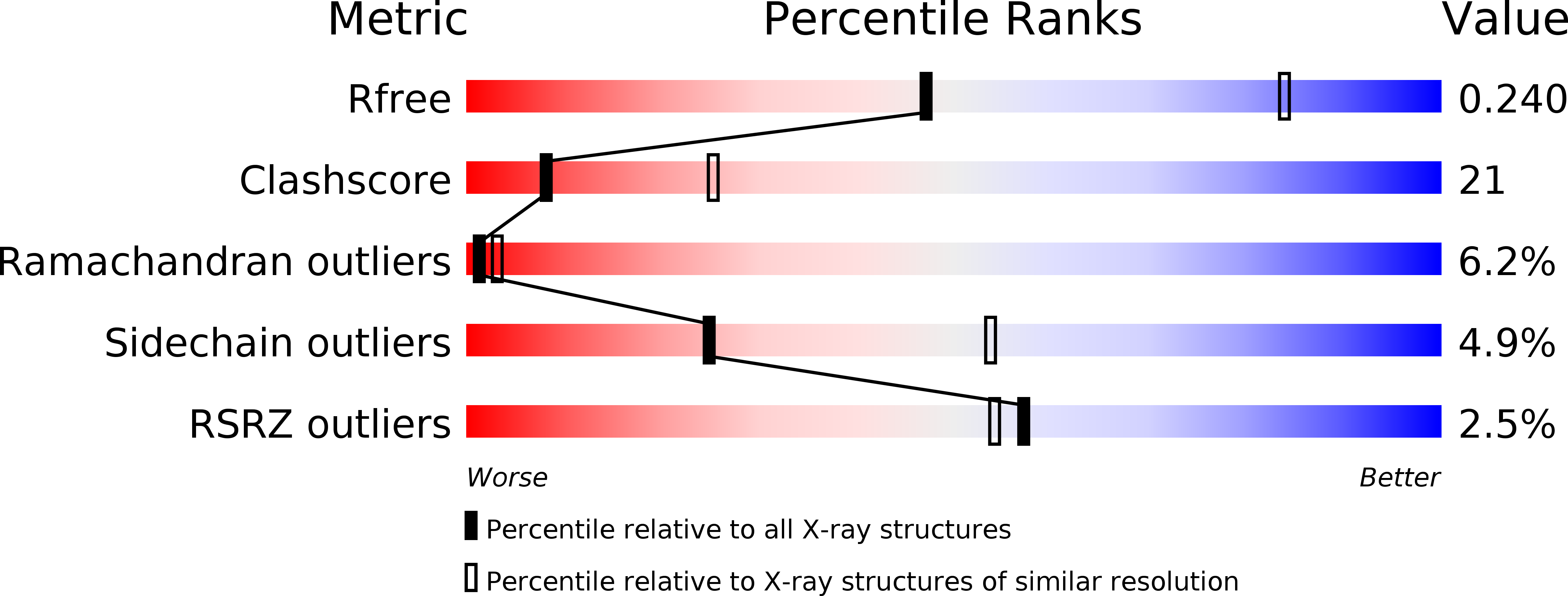
Investigation of high pressure effect on the structure and adsorption of beta-lactoglobulin.
Kurpiewska, K., Biela, A., Loch, J.I., Swiatek, S., Jachimska, B., Lewinski, K.(2017) Colloids Surf B Biointerfaces 161: 387-393
- PubMed: 29112912
- DOI: https://doi.org/10.1016/j.colsurfb.2017.10.069
- Primary Citation of Related Structures:
5IO5, 5IO6, 5IO7 - PubMed Abstract:
β-Lactoglobulin, being one of the principal whey protein, is of huge importance to the food industry. Temperature/pressure effects on this small protein has been extensively studied by industry. To characterize biochemical properties of β-lactoglobulin after or during pressurization, a wide range of methods have been used thus far. In this study, for the first time, the pressure-induced conformation of β-lactoglobulin in the crystal state was determined, at pressure 430 MPa. Changes observed in the high pressure structure correlate with the physico-chemical properties of pressure-treated β-lactoglobulin obtained from dynamic light scattering, electrophoretic mobility and quartz crystal microbalance with dissipation monitoring measurements. A comparison between the β-lactoglobulin structures determined at both high and ambient pressure contrasts the stable nature of the protein core and adjacent loop fragments. At high pressure the β-lactoglobulin structure presents early signs of dimer dissociation, charge and conformational changes characteristic for initial unfolded intermediate as well as a significant modification of the binding pocket volume. Those observations are supported by changes in zeta potential values and results in increase affinity of the β-lactoglobulin adsorption onto gold surface. Observed pressure-induced structural modifications were previously suggested as an important factor contributing to β-lactoglobulin denaturation process. Presented studies provide detailed analysis of pressure-associated structural changes influencing β-lactoglobulin conformation and consequently its adsorption.
Organizational Affiliation:
Jagiellonian University, Faculty of Chemistry, Department of Crystal Chemistry and Crystal Physics, Biocrystallography Group, Gronostajowa 2, 30-387, Kraków, Poland; Jerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences, Niezapominajek 8, 30-239 Kraków, Poland. Electronic address: kurpiews@chemia.uj.edu.pl.