Tet3 Reads 5-Carboxylcytosine through Its CXXC Domain and Is a Potential Guardian against Neurodegeneration.
Jin, S.G., Zhang, Z.M., Dunwell, T.L., Harter, M.R., Wu, X., Johnson, J., Li, Z., Liu, J., Szabo, P.E., Lu, Q., Xu, G.L., Song, J., Pfeifer, G.P.(2016) Cell Rep 14: 493-505
- PubMed: 26774490
- DOI: https://doi.org/10.1016/j.celrep.2015.12.044
- Primary Citation of Related Structures:
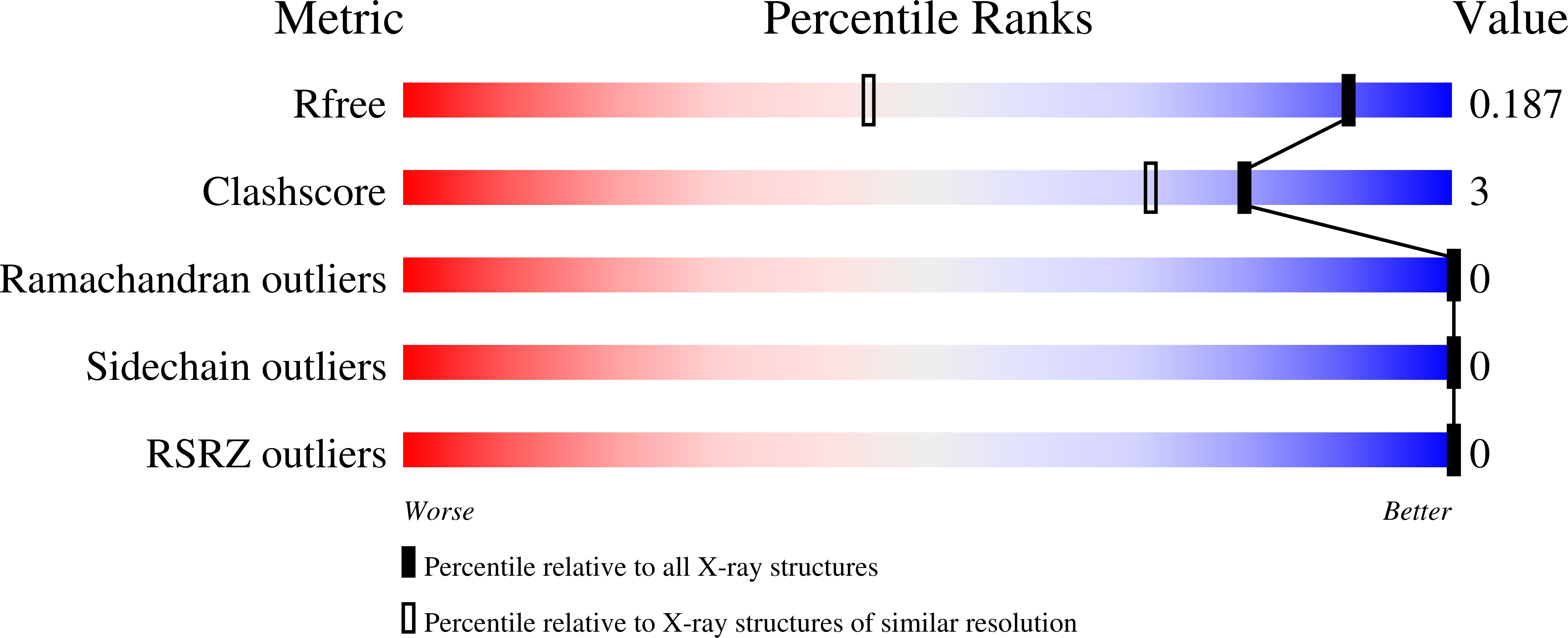
5EXH - PubMed Abstract:
We report that the mammalian 5-methylcytosine (5mC) oxidase Tet3 exists as three major isoforms and characterized the full-length isoform containing an N-terminal CXXC domain (Tet3FL). This CXXC domain binds to unmethylated CpGs, but, unexpectedly, its highest affinity is toward 5-carboxylcytosine (5caC). We determined the crystal structure of the CXXC domain-5caC-DNA complex, revealing the structural basis of the binding specificity of this domain as a reader of CcaCG sequences. Mapping of Tet3FL in neuronal cells shows that Tet3FL is localized precisely at the transcription start sites (TSSs) of genes involved in lysosome function, mRNA processing, and key genes of the base excision repair pathway. Therefore, Tet3FL may function as a regulator of 5caC removal by base excision repair. Active removal of accumulating 5mC from the TSSs of genes coding for lysosomal proteins by Tet3FL in postmitotic neurons of the brain may be important for preventing neurodegenerative diseases.
Organizational Affiliation:
Center for Epigenetics, Van Andel Research Institute, Grand Rapids, MI 49503, USA; Department of Cancer Biology, Beckman Research Institute of the City of Hope, Duarte, CA 91010, USA.