Response to Comment on "Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism".
Li, F., Liu, J., Zheng, Y., Garavito, R.M., Ferguson-Miller, S.(2015) Science 350: 519-519
- PubMed: 26516277
- DOI: https://doi.org/10.1126/science.aab2595
- Primary Citation of Related Structures:
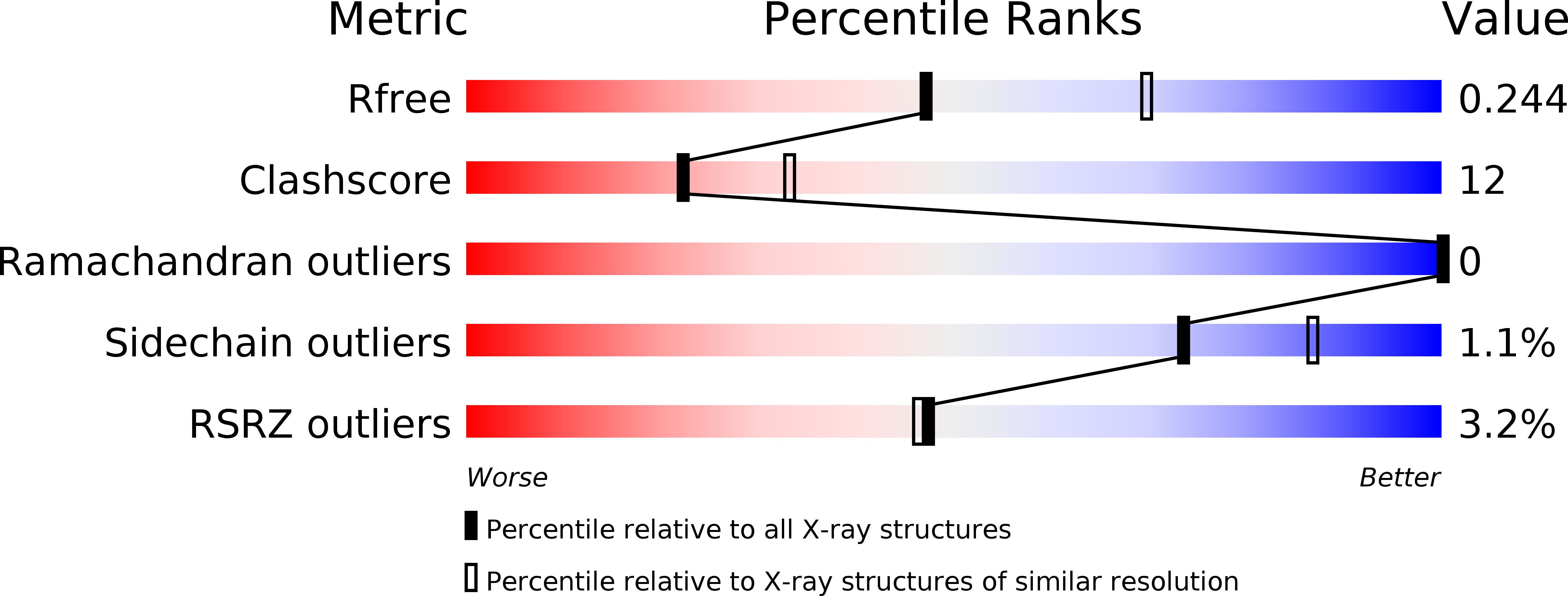
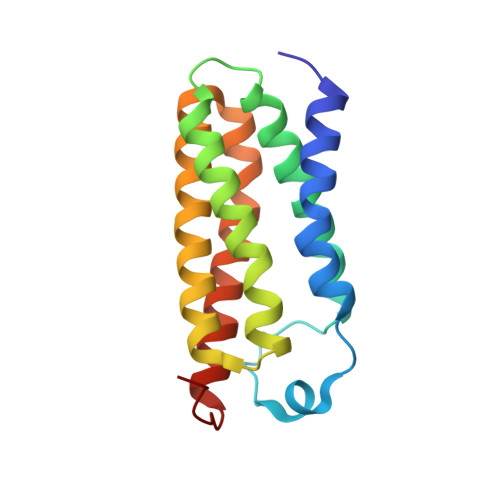
5DUO - PubMed Abstract:
Wang comments that the diffraction data for the structure of the A139T mutant of translocator protein TSPO from Rhodobacter sphaeroides should be used to 1.65 instead of 1.8 angstroms and that the density interpreted as porphyrin and monoolein is better fitted as polyethylene glycol. Although different practices of data processing exist, in this case they do not substantially influence the final map. Additional data are presented supporting the fit of a porphyrin and monooleins.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, USA.