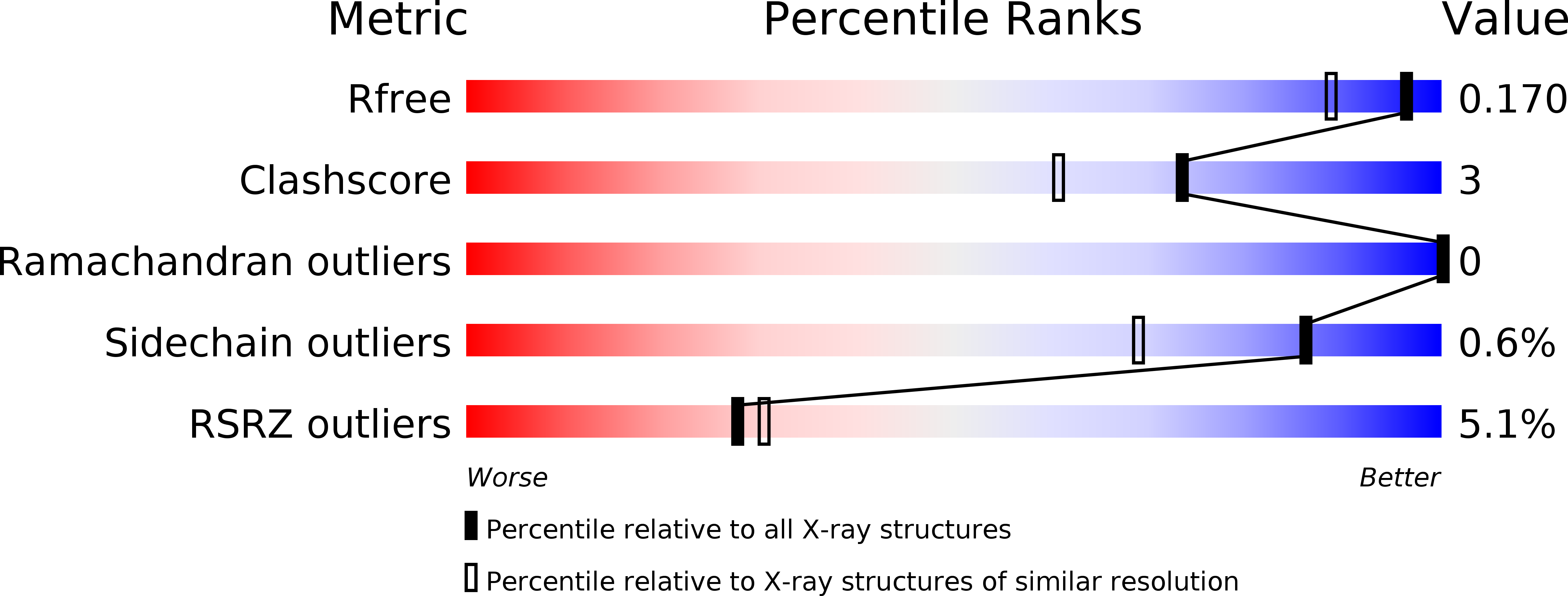
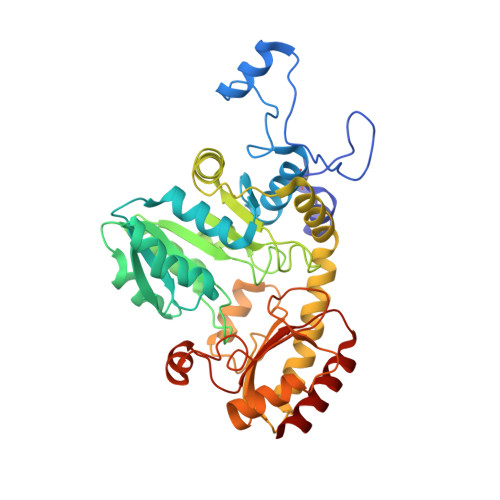
Crystal structure of mutant form Cys115His of Citrobacter freundii methionine gamma-lyase complexed with l-norleucine.
Revtovich, S.V., Morozova, E.A., Kulikova, V.V., Anufrieva, N.V., Osipova, T.I., Koval, V.S., Nikulin, A.D., Demidkina, T.V.(2017) Biochim Biophys Acta 1865: 1123-1128
- PubMed: 28602917
- DOI: https://doi.org/10.1016/j.bbapap.2017.06.001
- Primary Citation of Related Structures:
5M3Z - PubMed Abstract:
The mutant form of Citrobacter freundii methionine γ-lyase with the replacement of active site Cys115 for His has been found to be inactive in the γ-elimination reaction of methionine while fully active in the γ-elimination reaction of O-acetyl-l-homoserine and in the β-elimination reaction of S-alk(en)yl-substituted cysteines. In this work, the crystal structure of the mutant enzyme complexed with competitive inhibitor, l-norleucine was determined at 1.45Å resolution. At the enzyme active site the inhibitor proved to be bound both noncovalently and covalently, which corresponds to the two intermediates of the γ- and β-elimination reactions, Michaelis complex and the external aldimine. Analysis of the structure allowed us to suggest the possible reason for the inability of the mutant enzyme to catalyze the physiological reaction.
Organizational Affiliation:
Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia.