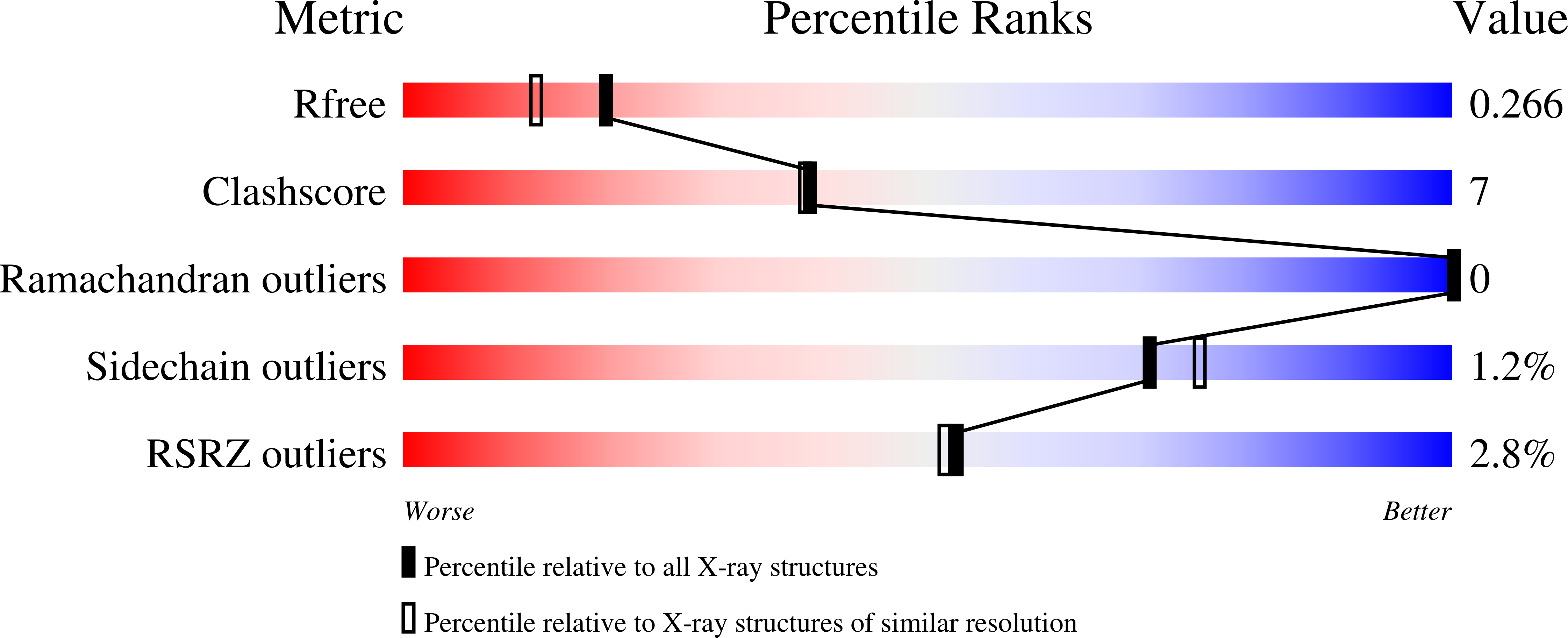
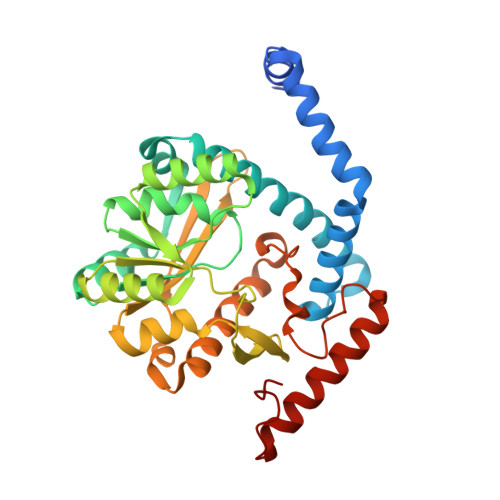
Structure-guided enzymology of the lipid A acyltransferase LpxM reveals a dual activity mechanism.
Dovala, D., Rath, C.M., Hu, Q., Sawyer, W.S., Shia, S., Elling, R.A., Knapp, M.S., Metzger, L.E.(2016) Proc Natl Acad Sci U S A 113: E6064-E6071
- PubMed: 27681620
- DOI: https://doi.org/10.1073/pnas.1610746113
- Primary Citation of Related Structures:
5KN7, 5KNK - PubMed Abstract:
Gram-negative bacteria possess a characteristic outer membrane, of which the lipid A constituent elicits a strong host immune response through the Toll-like receptor 4 complex, and acts as a component of the permeability barrier to prevent uptake of bactericidal compounds. Lipid A species comprise the bulk of the outer leaflet of the outer membrane and are produced through a multistep biosynthetic pathway conserved in most Gram-negative bacteria. The final steps in this pathway involve the secondary acylation of lipid A precursors. These are catalyzed by members of a superfamily of enzymes known as lysophospholipid acyltransferases (LPLATs), which are present in all domains of life and play important roles in diverse biological processes. To date, characterization of this clinically important class of enzymes has been limited by a lack of structural information and the availability of only low-throughput biochemical assays. In this work, we present the structure of the bacterial LPLAT protein LpxM, and we describe a high-throughput, label-free mass spectrometric assay to characterize acyltransferase enzymatic activity. Using our structure and assay, we identify an LPLAT thioesterase activity, and we provide experimental evidence to support an ordered-binding and "reset" mechanistic model for LpxM function. This work enables the interrogation of other bacterial acyltransferases' structure-mechanism relationships, and the assay described herein provides a foundation for quantitatively characterizing the enzymology of any number of clinically relevant LPLAT proteins.
Organizational Affiliation:
Infectious Diseases, Novartis Institutes for BioMedical Research, Emeryville, CA 94608.