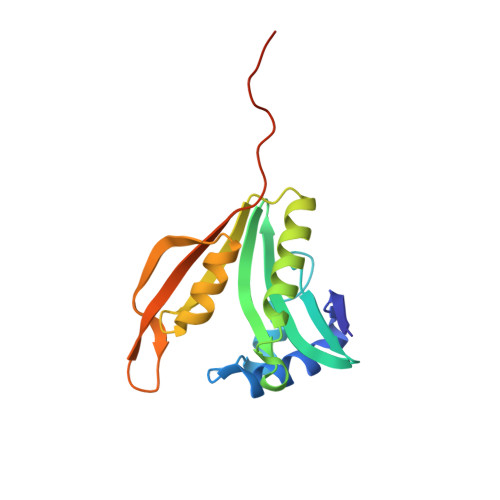
Crystal structure of the ribosomal-protein-S18-alanine N-acetyltransferase from Escherichia coli
Filippova, E.V., Minasov, G., Kiryukhina, O., Shuvalova, L., Grimshaw, S., Wolfe, A.J., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.