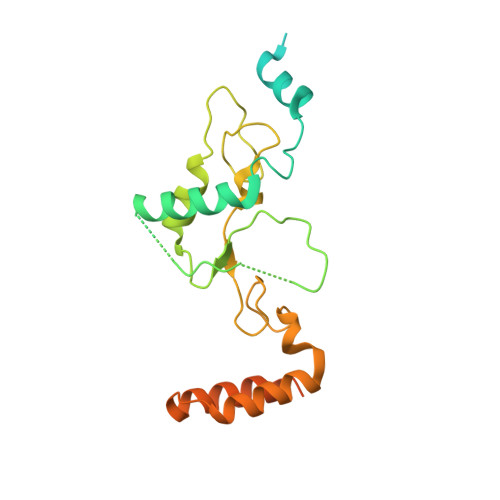
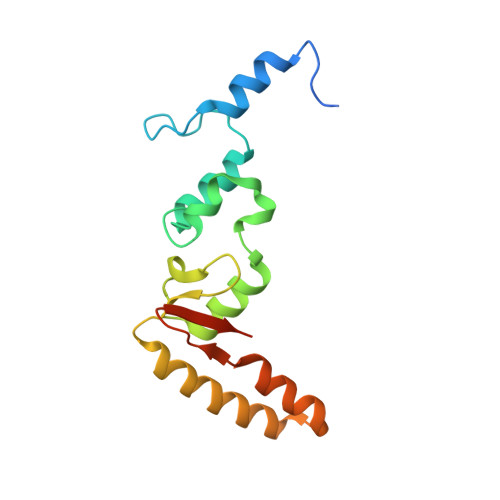
SHREC Silences Heterochromatin via Distinct Remodeling and Deacetylation Modules.
Job, G., Brugger, C., Xu, T., Lowe, B.R., Pfister, Y., Qu, C., Shanker, S., Banos Sanz, J.I., Partridge, J.F., Schalch, T.(2016) Mol Cell 62: 207-221
- PubMed: 27105116
- DOI: https://doi.org/10.1016/j.molcel.2016.03.016
- Primary Citation of Related Structures:
5IKF, 5IKJ, 5IKK - PubMed Abstract:
Nucleosome remodeling and deacetylation (NuRD) complexes are co-transcriptional regulators implicated in differentiation, development, and diseases. Methyl-CpG binding domain (MBD) proteins play an essential role in recruitment of NuRD complexes to their target sites in chromatin. The related SHREC complex in fission yeast drives transcriptional gene silencing in heterochromatin through cooperation with HP1 proteins. How remodeler and histone deacetylase (HDAC) cooperate within NuRD complexes remains unresolved. We determined that in SHREC the two modules occupy distant sites on the scaffold protein Clr1 and that repressive activity of SHREC can be modulated by the expression level of the HDAC-associated Clr1 domain alone. Moreover, the crystal structure of Clr2 reveals an MBD-like domain mediating recruitment of the HDAC module to heterochromatin. Thus, SHREC bi-functionality is organized in two separate modules with separate recruitment mechanisms, which work together to elicit transcriptional silencing at heterochromatic loci.
Organizational Affiliation:
Department of Pathology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, USA.