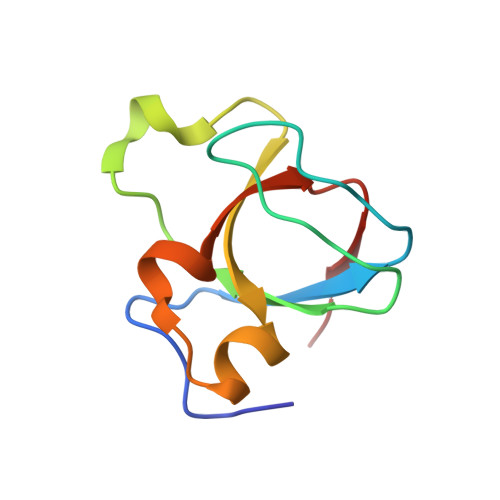
Structural Basis of the High Affinity Interaction between the Alphavirus Nonstructural Protein-3 (nsP3) and the SH3 Domain of Amphiphysin-2.
Tossavainen, H., Aitio, O., Hellman, M., Saksela, K., Permi, P.(2016) J Biol Chem 291: 16307-16317
- PubMed: 27268056
- DOI: https://doi.org/10.1074/jbc.M116.732412
- Primary Citation of Related Structures:
5I22 - PubMed Abstract:
We show that a peptide from Chikungunya virus nsP3 protein spanning residues 1728-1744 binds the amphiphysin-2 (BIN1) Src homology-3 (SH3) domain with an unusually high affinity (Kd 24 nm). Our NMR solution complex structure together with isothermal titration calorimetry data on several related viral and cellular peptide ligands reveal that this exceptional affinity originates from interactions between multiple basic residues in the target peptide and the extensive negatively charged binding surface of amphiphysin-2 SH3. Remarkably, these arginines show no fixed conformation in the complex structure, indicating that a transient or fluctuating polyelectrostatic interaction accounts for this affinity. Thus, via optimization of such dynamic electrostatic forces, viral peptides have evolved a superior binding affinity for amphiphysin-2 SH3 compared with typical cellular ligands, such as dynamin, thereby enabling hijacking of amphiphysin-2 SH3-regulated host cell processes by these viruses. Moreover, our data show that the previously described consensus sequence PXRPXR for amphiphysin SH3 ligands is inaccurate and instead define it as an extended Class II binding motif PXXPXRpXR, where additional positive charges between the two constant arginine residues can give rise to extraordinary high SH3 binding affinity.
Organizational Affiliation:
From the Program in Structural Biology and Biophysics, Institute of Biotechnology, University of Helsinki and.