Crystal structures of the bifunctional tRNA methyltransferase Trm5a
Wang, C., Jia, Q., Chen, R., Wei, Y., Li, J., Ma, J., Xie, W.(2016) Sci Rep 6: 33553-33553
- PubMed: 27629654
- DOI: https://doi.org/10.1038/srep33553
- Primary Citation of Related Structures:
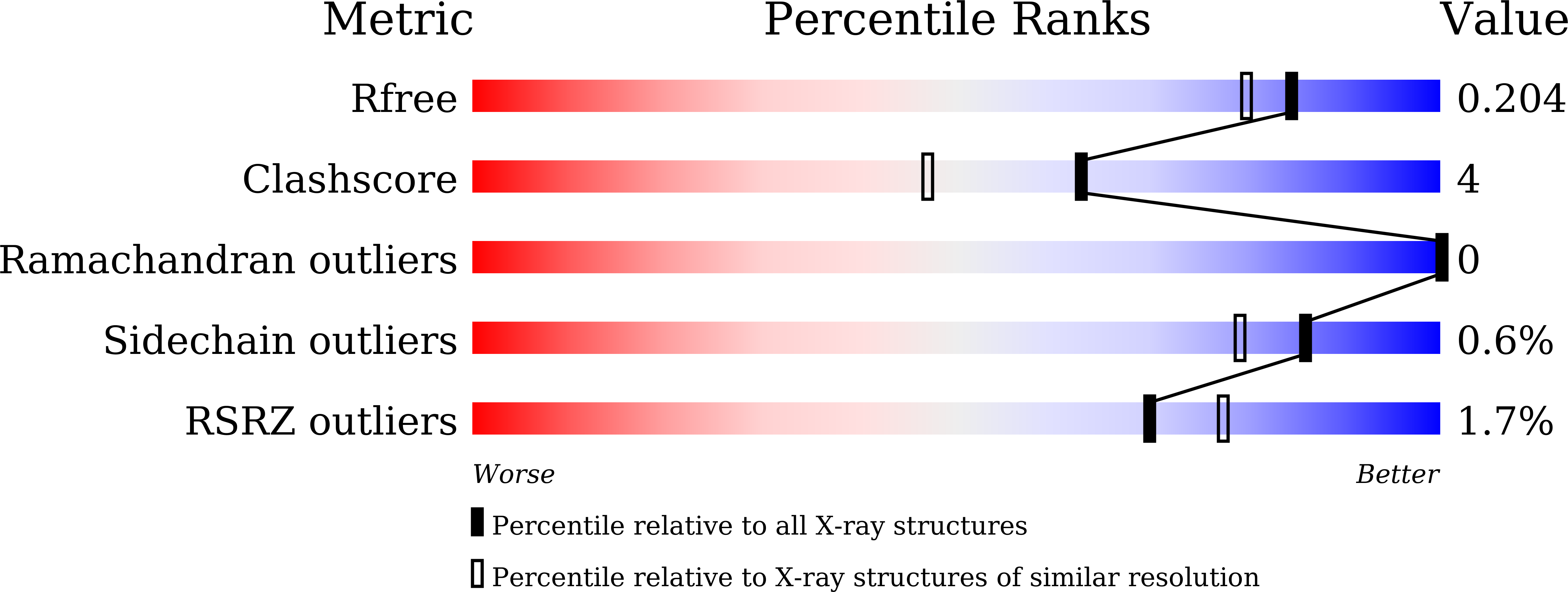
5HJI, 5HJJ, 5HJK, 5HJM - PubMed Abstract:
tRNA methyltransferase Trm5 catalyses the transfer of a methyl group from S-adenosyl-L-methionine to G37 in eukaryotes and archaea. The N1-methylated guanosine is the product of the initial step of the wyosine hypermodification, which is essential for the maintenance of the reading frame during translation. As a unique member of this enzyme family, Trm5a from Pyrococcus abyssi (PaTrm5a) catalyses not only the methylation of N1, but also the further methylation of C7 on 4-demethylwyosine at position 37 to produce isowyosine, but the mechanism for the double methylation is poorly understood. Here we report four crystal structures of PaTrm5a ranging from 1.7- to 2.3-Å, in the apo form or in complex with various SAM analogues. These structures reveal that Asp243 specifically recognises the base moiety of SAM at the active site. Interestingly, the protein in our structures all displays an extended conformation, quite different from the well-folded conformation of Trm5b from Methanocaldococcus jannaschii reported previously, despite their similar overall architectures. To rule out the possibilities of crystallisation artefacts, we conducted the fluorescence resonance energy transfer (FRET) experiments. The FRET data suggested that PaTrm5a adopts a naturally extended conformation in solution, and therefore the open conformation is a genuine state of PaTrm5a.
Organizational Affiliation:
State Key Laboratory for Biocontrol, School of Life Sciences, The Sun Yat-Sen University, Guangzhou, Guangdong 510275, People's Republic of China.